- Hutchmed
- | 公告及新闻稿
London: Friday, June 29, 2018: Hutchison China MediTech Limited (“Chi-Med”) (AIM/Nasdaq: HCM) hereby notifies the market that as at June 29, 2018, the issued share capital of Chi-Med consisted of 66,532,683 ordinary shares of US$1.00 each, with each share carrying one right to vote and with no shares held in treasury.
The above figure of 66,532,683 may be used by shareholders as the denominator for the calculations by which they could determine if they are required to notify their interest in, or a change to their interest in, Chi-Med under the Financial Conduct Authority’s Disclosure Rules and Transparency Rules.
For illustrative purposes only, the 66,532,683 ordinary shares would be equivalent to 66,532,683 CREST depositary interests (each equating to one ordinary share) which are traded on AIM or, if the CREST depositary interests were converted in their entirety, equivalent to 133,065,366 American depositary shares (each equating to one-half of one ordinary share) which are traded on Nasdaq.
About Chi-Med
Chi-Med is an innovative biopharmaceutical company which researches, develops, manufactures and sells pharmaceuticals and healthcare products. Its Innovation Platform, Hutchison MediPharma Limited, focuses on discovering and developing innovative therapeutics in oncology and autoimmune diseases for the global market. Its Commercial Platform manufactures, markets, and distributes prescription drugs and consumer health products in China.
Chi-Med is majority owned by the multinational conglomerate CK Hutchison Holdings Limited (SEHK: 1). For more information, please visit: www.chi-med.com.
CONTACTS
Investor Enquiries
Mark Lee, Senior Vice President,
Corporate Finance & Development
+852 2121 8200
U.K. & International Media Enquiries
Anthony Carlisle, Citigate Dewe Rogerson
+44 7973 611 888 (Mobile)
anthony.carlisle@cdrconsultancy.co.uk
U.S. Based Media Enquiries
Brad Miles, Solebury Trout
+1 (917) 570 7340 (Mobile)
bmiles@troutgroup.com
Susan Duffy, Solebury Trout
+1 (917) 499 8887 (Mobile)
sduffy@troutgroup.com
Investor Relations
Xuan Yang, Solebury Trout
+1 (415) 971 9412 (Mobile)
xyang@troutgroup.com
David Dible, Citigate Dewe Rogerson
+44 7967 566 919 (Mobile)
david.dible@citigatedewerogerson.com
Panmure Gordon (UK) Limited
Richard Gray / Andrew Potts
+44 (20) 7886 2500
London: Friday, June 29, 2018: Hutchison China MediTech Limited (“Chi-Med”) (AIM/Nasdaq: HCM) announces the following blocklisting six monthly return:
| 1. | Name of applicant: | Hutchison China MediTech Limited | |
| 2. | Name of scheme: | (a) | Share Option Scheme conditionally adopted by Hutchison China MediTech Limited in 2005 (“2005 HCML Share Option Scheme”) |
| (b) | Share Option Scheme conditionally adopted by Hutchison China MediTech Limited in 2015 (“2015 HCML Share Option Scheme”) | ||
| 3. | Period of return: | From December 29, 2017 to June 28, 2018 | |
| 4. | Balance under scheme from previous return: | (a) | 2005 HCML Share Option Scheme: 304,999 ordinary shares of US$1 each |
| (b) | 2015 HCML Share Option Scheme: 1,000,000 ordinary shares of US$1 each | ||
| 5. | The amount by which the block scheme has been increased, if the scheme has been increased since the date of the last return: | (a) | 2005 HCML Share Option Scheme: Nil |
| (b) | 2015 HCML Share Option Scheme: 1,425,597 ordinary shares of US$1 each | ||
| 6. | Number of securities issued/allotted under scheme during period: | (a) | 2005 HCML Share Option Scheme: 85,646 ordinary shares of US$1 each |
| (b) | 2015 HCML Share Option Scheme: Nil ordinary shares of US$1 each | ||
| 7. | Balance under scheme not yet issued/allotted at end of the period: | (a) | 2005 HCML Share Option Scheme: 219,353 ordinary shares of US$1 each |
| (b) | 2015 HCML Share Option Scheme: 2,425,597 ordinary shares of US$1 each | ||
| 8. | Number and class of securities originally listed and the date of admission: | (i) | 2,560,606 ordinary shares of US$1 each admitted on June 26, 2007 |
| (ii) | 1,000,000 ordinary shares of US$1 each admitted on June 20, 2016 | ||
| (iii) | 1,425,597 ordinary shares of US$1 each admitted on April 27, 2018 | ||
| 9. | Total number of securities in issue at the end of the period: | 66,532,683 ordinary shares of US$1 each | |
| Name of contact: | Christian Hogg | ||
| Address of contact: | 21/F., Hutchison House, 10 Harcourt Road, Hong Kong | ||
| Telephone number of contact: | +852 2121 8200 | ||
About Chi-Med
Chi-Med is an innovative biopharmaceutical company which researches, develops, manufactures and sells pharmaceuticals and healthcare products. Its Innovation Platform, Hutchison MediPharma Limited, focuses on discovering and developing innovative therapeutics in oncology and autoimmune diseases for the global market. Its Commercial Platform manufactures, markets, and distributes prescription drugs and consumer health products in China.
Chi-Med is majority owned by the multinational conglomerate CK Hutchison Holdings Limited (SEHK: 1). For more information, please visit: www.chi-med.com.
CONTACTS
Investor Enquiries
Mark Lee, Senior Vice President,
Corporate Finance & Development
+852 2121 8200
U.K. & International Media Enquiries
Anthony Carlisle, Citigate Dewe Rogerson
+44 7973 611 888 (Mobile)
anthony.carlisle@cdrconsultancy.co.uk
U.S. Based Media Enquiries
Brad Miles, Solebury Trout
+1 (917) 570 7340 (Mobile)
bmiles@troutgroup.com
Susan Duffy, Solebury Trout
+1 (917) 499 8887 (Mobile)
sduffy@troutgroup.com
Investor Relations
Xuan Yang, Solebury Trout
+1 (415) 971 9412 (Mobile)
xyang@troutgroup.com
David Dible, Citigate Dewe Rogerson
+44 7967 566 919 (Mobile)
david.dible@citigatedewerogerson.com
Panmure Gordon (UK) Limited
Richard Gray / Andrew Potts
+44 (20) 7886 2500
London: Friday, June 29, 2018: Hutchison China MediTech Limited (“Chi-Med”) (AIM/Nasdaq: HCM) will be announcing its interim results for the six months ended June 30, 2018 on Friday, July 27, 2018 at 7:00 am British Summer Time (BST).
An analyst presentation will be held at 9:00 am BST (4:00 pm Hong Kong Time) on the same day at Citigate Dewe Rogerson, 3 London Wall Buildings, London, EC2M 5SY, UK, which will be webcast via the company website at www.chi-med.com/investors/event-information. The presentation will be available to download before the analyst presentation begins.
For North America based analysts and investors, Chi-Med will also host a conference call with Q&A at 9:00 am Eastern Daylight Time (2:00 pm BST).
Details of the analyst presentation and conference call dial-in will be provided in the financial results announcement. A replay will also be available on the website shortly after each event.
About Chi-Med
Chi-Med is an innovative biopharmaceutical company which researches, develops, manufactures and sells pharmaceuticals and healthcare products. Its Innovation Platform, Hutchison MediPharma Limited, focuses on discovering and developing innovative therapeutics in oncology and autoimmune diseases for the global market. Its Commercial Platform manufactures, markets, and distributes prescription drugs and consumer health products in China.
Chi-Med is majority owned by the multinational conglomerate CK Hutchison Holdings Limited (SEHK: 1). For more information, please visit: www.chi-med.com.
CONTACTS
Investor Enquiries
Mark Lee, Senior Vice President,
Corporate Finance & Development
+852 2121 8200
U.K. & International Media Enquiries
Anthony Carlisle, Citigate Dewe Rogerson
+44 7973 611 888 (Mobile)
anthony.carlisle@cdrconsultancy.co.uk
U.S. Based Media Enquiries
Brad Miles, Solebury Trout
+1 (917) 570 7340 (Mobile)
bmiles@troutgroup.com
Susan Duffy, Solebury Trout
+1 (917) 499 8887 (Mobile)
sduffy@troutgroup.com
Investor Relations
Xuan Yang, Solebury Trout
+1 (415) 971 9412 (Mobile)
xyang@troutgroup.com
David Dible, Citigate Dewe Rogerson
+44 7967 566 919 (Mobile)
david.dible@citigatedewerogerson.com
Panmure Gordon (UK) Limited
Richard Gray / Andrew Potts
+44 (20) 7886 2500
Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer
The FRESCO Randomized Clinical Trial
 Jin Li; Shukui Qin; Rui-Hua Xu; Lin Shen; Jianming Xu; Yuxian Bai; Lei Yang; Yanhong Deng; Zhen-dong Chen; Haijun Zhong; Hongming Pan; Weijian Guo; Yongqian Shu; Ying Yuan; Jianfeng Zhou; Nong Xu; Tianshu Liu; Dong Ma; Changping Wu; Ying Cheng; Donghui Chen; Wei Li; Sanyuan Sun; Zhuang Yu; Peiguo Cao; Haihui Chen; Jiejun Wang; Shubin Wang; Hongbing Wang; Songhua Fan; Ye Hua; Weiguo Su
Jin Li; Shukui Qin; Rui-Hua Xu; Lin Shen; Jianming Xu; Yuxian Bai; Lei Yang; Yanhong Deng; Zhen-dong Chen; Haijun Zhong; Hongming Pan; Weijian Guo; Yongqian Shu; Ying Yuan; Jianfeng Zhou; Nong Xu; Tianshu Liu; Dong Ma; Changping Wu; Ying Cheng; Donghui Chen; Wei Li; Sanyuan Sun; Zhuang Yu; Peiguo Cao; Haihui Chen; Jiejun Wang; Shubin Wang; Hongbing Wang; Songhua Fan; Ye Hua; Weiguo Su
Abstract
Importance
Patients with metastatic colorectal cancer (CRC) have limited effective and tolerable treatment options.
Objective
To evaluate the efficacy and safety of oral fruquintinib, a vascular endothelial growth factor receptor (VEGFR) inhibitor, as third-line or later therapy in patients with metastatic CRC.
Design, Setting, and Participants
FRESCO (Fruquintinib Efficacy and Safety in 3+ Line Colorectal Cancer Patients) was a randomized, double-blind, placebo-controlled, multicenter (28 hospitals in China), phase 3 clinical trial. From December 2014 to May 2016, screening took place among 519 patients aged 18 to 75 years who had metastatic CRC that progressed after at least 2 lines of chemotherapy but had not received VEGFR inhibitor therapy; 416 met the eligibility criteria and were stratified by prior anti-VEGF therapy and K-ras status. The final date of follow-up was January 17, 2017.
Interventions
Patients were randomized in a 2:1 ratio to receive either fruquintinib, 5 mg (n = 278) or placebo (n = 138) orally, once daily for 21 days, followed by 7 days off in 28-day cycles, until disease progression, intolerable toxicity, or study withdrawal.
Main Outcomes and Measures
The primary end point was overall survival. Key secondary efficacy endpoints were progression-free survival (time from randomization to disease progression or death), objectiveresponse rate (confirmed complete or partial response), and disease control rate (complete or partial response, or stabledisease recorded ≥8 weeks postrandomization). Duration of response was also assessed. Safety outcomes included treatment-emergent adverse events.
Results
Of the 416 randomized patients (mean age, 54.6 years; 161 [38.7%] women), 404 (97.1%) completed the trial. Median overall survival was significantly prolonged with fruquintinib compared with placebo (9.3 months [95% CI, 8.2-10.5] vs 6.6 months [95% CI, 5.9-8.1]); hazard ratio (HR) for death, 0.65 (95% CI, 0.51-0.83; P < .001). Median progression-free survival was also significantly increased with fruquintinib (3.7 months [95% CI, 3.7-4.6] vs 1.8 months [95% CI, 1.8-1.8] months); HR for progression or death, 0.26 (95% CI, 0.21 to 0.34; P < .001). Grades 3 and 4 treatment-emergent adverse events occurred in 61.2% (170) of patients who received fruquintinib and 19.7% (27) who received placebo. Serious adverse events were reported by 15.5% (43) of patients in the fruquintinib group and 5.8% (8) in the placebo group, with 14.4% (40) of fruquintinib-treated and 5.1% (7) of placebo-treated patients requiring hospitalization.
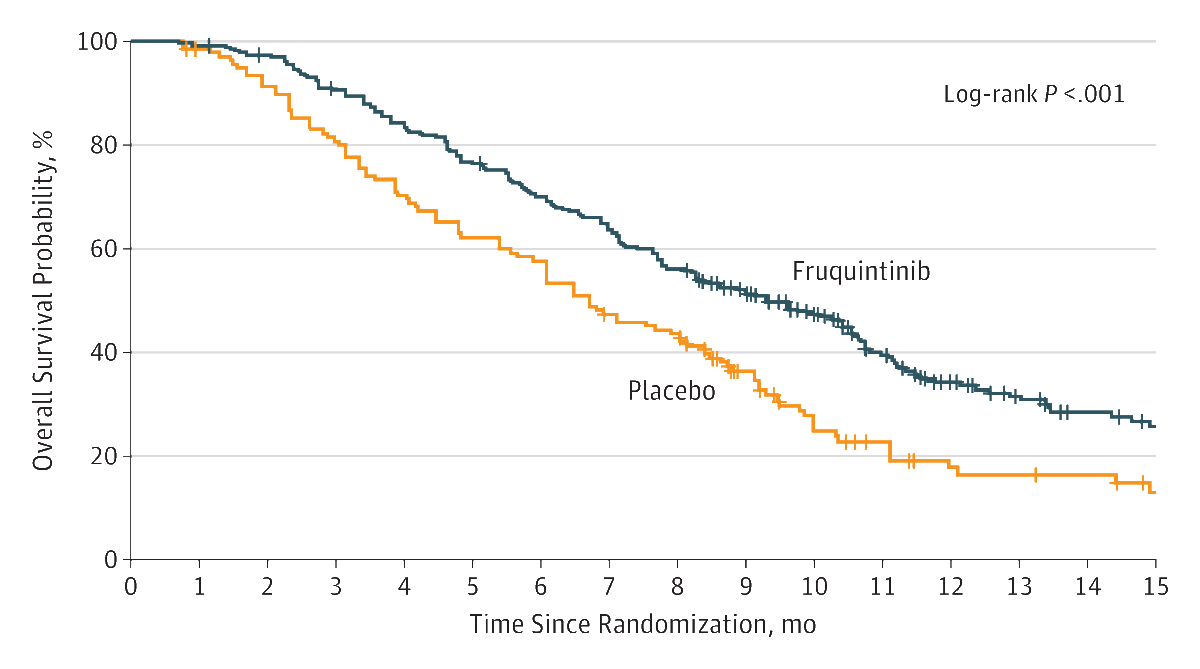
Conclusions and Relevance
Among Chinese patients with metastatic CRC who had tumor progression following at least 2 prior chemotherapy regimens, oral fruquintinib compared with placebo resulted in a statistically significant increase in overall survival. Further research is needed to assess efficacy outside of China.
Trial Registration ClinicalTrials.gov Identifier: NCT02314819
Citations and Links
Please follow the link below to access the publication:
JAMA. 2018;319(24):2486-2496.
London: Thursday, June 7, 2018: Hutchison China MediTech Limited (“Chi-Med”) (AIM/Nasdaq: HCM) announces that on June 6, 2018, it granted share options under the Share Option Scheme conditionally adopted by Chi-Med at its Annual General Meeting in 2015 (the “2015 HCML Share Option Scheme”).
Chi-Med granted 36,936 share options under its 2015 HCML Share Option Scheme to certain employees to subscribe for Ordinary Shares subject to the acceptance of the grantees. Details of such share options granted prescribed are as follows:
Date of grant: June 6, 2018
Exercise price of share options granted: GBP41.66 per Ordinary Share
Number of share options granted: 36,936 (each share option shall entitle the holder thereof to subscribe for one Ordinary Share)
Closing market price of Ordinary Shares on the date of grant: GBP41.40 per Ordinary Share
Validity period of the share options: From June 6, 2018 to June 5, 2028
About Chi-Med
Chi-Med is an innovative biopharmaceutical company which researches, develops, manufactures and sells pharmaceuticals and healthcare products. Its Innovation Platform, Hutchison MediPharma Limited, focuses on discovering and developing innovative therapeutics in oncology and autoimmune diseases for the global market. Its Commercial Platform manufactures, markets, and distributes prescription drugs and consumer health products in China.
Chi-Med is majority owned by the multinational conglomerate CK Hutchison Holdings Limited (SEHK: 1). For more information, please visit: www.chi-med.com.
Forward Looking Statements
This announcement contains forward-looking statements within the meaning of the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements involve risks and uncertainties. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see Chi-Med’s filings with the U.S. Securities and Exchange Commission and on AIM. Chi-Med undertakes no obligation to update or revise the information contained in this announcement, whether as a result of new information, future events or circumstances or otherwise.
CONTACTS
Investor Enquiries
Mark Lee, Senior Vice President,
Corporate Finance & Development
+852 2121 8200
U.K. & International Media Enquiries
Anthony Carlisle, Citigate Dewe Rogerson
+44 7973 611 888 (Mobile)
anthony.carlisle@cdrconsultancy.co.uk
U.S. Based Media Enquiries
Brad Miles, Solebury Trout
+1 (917) 570 7340 (Mobile)
bmiles@troutgroup.com
Susan Duffy, Solebury Trout
+1 (917) 499 8887 (Mobile)
sduffy@troutgroup.com
Investor Relations
Xuan Yang, Solebury Trout
+1 (415) 971 9412 (Mobile)
xyang@troutgroup.com
David Dible, Citigate Dewe Rogerson
+44 7967 566 919 (Mobile)
david.dible@citigatedewerogerson.com
Panmure Gordon (UK) Limited
Richard Gray / Andrew Potts
+44 (20) 7886 2500
–继2017年ASCO年会上就FRESCO研究达到全部研究终点,呋喹替尼安全性良好,较其他靶向疗法脱靶毒性更低等数据进行口头报告后,和黄医药在2018年ASCO年会上公布了FRESCO研究进一步的研究数据–
–亚组分析显示呋喹替尼带来的生存获益在主要亚组中均非常稳健–
– 生存质量调整的生存分析显示呋喹替尼可带来生存质量获益–
2018年6月4日:和黄中国医药科技(AIM/Nasdaq:HCM)子公司和黄医药今日宣布在2018年美国临床肿瘤学会(ASCO)年会上公布了III期临床试验FRESCO进一步的研究数据。2018年ASCO年会于6月1日至5日在美国伊利诺斯州芝加哥市举办。这次公布的数据来自FRESCO研究纳入的416名局部晚期或转移性结直肠癌(CRC)患者。
呋喹替尼是一种高选择性强效口服血管内皮生长因子受体(VEGFR)1,2及3的抑制剂。呋喹替尼的新药上市申请(NDA)已被中国国家药品监督管理局(CNDA)受理。其中,FRESCO研究数据也是NDA申报材料的一部分。此外,呋喹替尼在中国还在开展以肺癌(以三线肺癌为适应症的III期临床研究FALUCA以及呋喹替尼联合易瑞沙®以一线肺癌为适应症的II期临床研究)和胃癌(以二线胃癌为适应症的III期临床研究FRUTIGA)为适应症的多项临床研究。此外,呋喹替尼在美国的临床研究也正在进行中(I期桥接试验)。
这次公布的两篇摘要内容如下:
一项在中国转移性结直肠癌患者中对比呋喹替尼和安慰剂分别联合最佳支持治疗的随机、双盲、III期临床研究FRESCO研究的亚组分析结果:既往是否接受过抗VEGF或抗EGFR靶向治疗对研究结果的影响
报告人:徐瑞华
摘要其他作者:李进、白玉贤、邓艳红、杨磊、钟海钧、陈振东、潘宏铭、郭伟剑、束永前、袁瑛、徐建明、沈琳、王宁、王欣、迟海东、彭猛业、华烨、苏慰国、秦叔逵
报告时间及地点:6月3日(周日)美国中部夏令时间(CDT)08:00-11:30;A厅;壁报版编号:#30
小节:胃肠道(结直肠)肿瘤
摘要号及连接:#3537; abstracts.asco.org/214/AbstView_214_215579.html
在FRESCO研究中,呋喹替尼为中国的三线转移性CRC患者带来了统计学上具有显著意义且临床意义也较明显的获益。这项研究通过分析FRESCO研究中既往接受过靶向治疗(PTT)亚组和既往未接受过靶向治疗(无PTT)亚组来探索既往靶向治疗对呋喹替尼疗效和安全性的影响。该亚组分析结果显示,无论既往是否接受过靶向治疗,呋喹替尼均能为三线转移性CRC患者带来临床获益,且未观测到累积毒性。
在第20届中国临床肿瘤学会年会上公布的研究结果显示,呋喹替尼在各个亚组表现稳健,均能带来获益。在接受呋喹替尼治疗的278位患者中,有111位既往接受过靶向治疗。在PTT亚组中,呋喹替尼对比安慰剂可以显著地延长患者的总生存期(OS)(中位OS:7.69个月 vs 5.98个月;HR=0.63;p = 0.023)以及无进展生存期(PFS)(中位PFS:3.65个月 vs 1.84个月;HR = 0.24;p<0.001)。呋喹替尼也能为既往接受过抗-VEGF治疗的患者(N=84)带来OS(中位OS:7.20个月 vs 5.91个月;HR =0.68;p=0.066 )和PFS(中位PFS:3.48个月vs 1.84个月;HR=0.24;p<0.001)的获益。在无PTT亚组中,接受呋喹替尼治疗的患者的中位OS为10.35个月,接受安慰剂的患者的中位OS为6.93个月(HR=0.63;p=0.01);呋喹替尼治疗患者的中位PFS为3.81个月,而安慰剂组患者的中位PFS为1.84个月(HR=0.28;p<0.001)。
此次在ASCO年会上公布的数据显示在PTT亚组中,接受呋喹替尼治疗的患者未观察到累积性的治疗过程中出现的3级及以上不良事件。PTT亚组和无PTT亚组患者在治疗过程中出现的3级及以上不良事件发生率相似(61.3%和61.1%)。该亚组分析结果与之前公布的FRESCO研究意向治疗人群结果一致。
呋喹替尼治疗转移性结直肠癌的随机III期临床研究FRESCO研究中患者的生存质量调整的无症状且无毒性时间(Q-TWiST)
报告人:白玉贤
摘要其他作者:李洪燕、王宁、郭晓军、王伟、范颂华、徐建明、沈琳
报告时间及地点:6月3日(周日)美国中部夏令时间(CDT)08:00-11:30;A厅;壁报版编号:#37
小节:胃肠道(结直肠)肿瘤
摘要号及连接:#3544; abstracts.asco.org/214/AbstView_214_224293.html
这项特设(ad-hoc)研究通过分析FRESCO研究中呋喹替尼组和安慰剂组生存质量调整的无症状且无毒性时间(Q-TWiST)来对比两组患者生存质量调整的生存情况以及呋喹替尼在不同亚组中带来的Q-TWiST获益。Q-TWiST分析旨在从患者的角度来评估不同治疗手段相对的临床获益与风险,已在许多肿瘤治疗方案的评估中得到了广泛地应用。在Q-TWiST分析中,每个患者的生存时间都被划分为三个部分:TOX(疾病进展前伴有3级及以上不良事件的时间)、TWiST(无疾病症状且无3级及以上不良反应的时间)和REL(疾病进展或复发至死亡或随访结束的时间)。
接受呋喹替尼治疗的患者相对于接受安慰剂的患者获得了更长的Q-TWiST时间,且Q-TWiST获益与患者既往接受过的化疗线数和既往是否接受过抗-VEGF或抗-EGFR的靶向治疗无关。Q-TWiST的改善显示呋喹替尼能够为转移性CRC患者带来具有临床价值的生存质量获益。
更多ASCO年会相关信息请访问am.asco.org查看。
关于呋喹替尼
呋喹替尼(HMPL-013)是一种新型的高选择性小分子候选药物。临床研究证实:通过一日一次的口服剂量即可有效的抑制血管内皮生长因子受体(VEGFR),且脱靶毒性低于其他靶向疗法。呋喹替尼良好的耐受性以及无药物间相互作用的特性,为其与其他癌症疗法相联合提供了理论支持,例如当前正在进行的呋喹替尼联合化疗或其他靶向治疗的临床研究。VEGFR在肿瘤的血管生成中起到了至关重要的作用,抑制VEGFR可以阻断肿瘤新生血管形成,从而成为防止肿瘤增长和入侵的一种重要的治疗策略。
关于呋喹替尼治疗结直肠癌在中国的研发进展
2017年6月,CNDA(前“国家食品药品监督管理局”)受理呋喹替尼以晚期结直肠癌(CRC)为适应症的新药上市申请。2017年9月,CNDA公布因呋喹替尼具有明显临床价值而授予其优先审评的资格。呋喹替尼的新药上市申请基于已经获得成功的FRESCO研究的研究数据。该研究结果于2017年6月5日在ASCO年会上以口头报告的形式公布。该研究详情可登录clinicaltrials.gov,检索NCT02314819查看。呋喹替尼的3项早期临床研究为FRESCO研究的开展奠定了基础,这3项早期临床研究包括:纳入40名实体瘤患者的I期临床研究、纳入62名结直肠癌患者的Ib期临床研究以及纳入71名结直肠癌患者的II期临床研究。
其他呋喹替尼相关研发项目
肺癌:呋喹替尼以非小细胞肺癌(NSCLC)为适应症的III期注册性临床试验FALUCA目前也正在中国展开。FALUCA是一项随机双盲安慰剂对照的多中心临床研究,目标受试者为二线系统化疗失败的晚期非鳞NSCLC患者。该研究患者入组工作已于2018年2月完成,共计纳入527名患者(clinicaltrials.gov注册号NCT02691299)。该研究基于一项设计相似、纳入了91名三线NSCLC患者的II期临床试验。这项II期临床试验的结果已于2016年12月6日举办的第17届世界肺癌大会上以口头报告的形式公布(clinicaltrials.gov 注册号 NCT02590965)。
和FALUCA同时进行的还有另外一项II期临床试验。该试验以呋喹替尼联合易瑞沙®(吉非替尼)作为一线疗法,治疗晚期或转移性NSCLC(clinicaltrials.gov 注册号 NCT02976116)。该试验的初步结果已于2017年10月16日举办的第18届世界肺癌大会上以口头报告的形式公布。
胃癌:2017年10月,和黄医药启动了呋喹替尼联合紫杉醇(泰素®)以晚期胃癌或胃食管结合部(GEJ)腺癌为适应症的关键性III期临床研究,这项研究被命名为FRUTIGA 。该研究是一项随机双盲安慰剂对照的多中心临床研究,计划纳入500余名患者,目标受试者为一线标准化疗后进展的晚期胃癌或GEJ腺癌患者(clinicaltrials.gov 注册号 NCT03223376)。FRUTIGA是在一项Ib/II期临床试验的基础上开展的,该试验表明呋喹替尼联合泰素®在这类患者中具有良好的耐受性和令人鼓舞的肿瘤缓解率(clinicaltrials.gov 注册号 NCT02415023)。
在中国范围内,呋喹替尼由和黄医药和礼来合作开发。
美国桥接试验:和黄医药于2017年12月在美国启动一项多中心开放标签的I期临床试验,旨在评估呋喹替尼在美国晚期实体瘤患者中的安全性、耐受性和药代动力学特性(clinicaltrials.gov 注册号NCT03251378)。
Quality-adjusted time without symptoms or toxicity (Q-TWiST) of patients with metastatic colorectal cancer treated with fruquintinib in FRESCO
| Presenter: | Yu-Xian Bai |
| Other Authors: | Hongyan Li, Ning Wang, Xiaojun Guo, Wei Wang, Songhua Fan, Jian-Ming Xu, Lin Shen |
| Time & Location: | Sunday, June 3, 08:00 – 11:30 CDT; Hall A, Poster Board: #37 |
| Session: | Gastrointestinal (Colorectal) Cancer |
| Abstract No. & Link: | #3544; abstracts.asco.org/214/AbstView_214_224293.html |
Copies of this poster can be obtained from American Society of Clinical Oncology and the author of the poster.
| Presenter: | Ruihua Xu |
| Other Authors: | Jin Li, Yu-Xian Bai, Yanhong Deng, Lei Yang, Haijun Zhong, Zhendong Chen, Hongming Pan, Weijian Guo, Yongqian Shu, Ying Yuan, Jianming Xu, Lin Shen, Ning Wang, Xin Wang, Haidong Chi, Jack Peng, Ye Hua, Weiguo Su, Shukui Qin |
| Time & Location: | Sunday, June 3, 08:00 – 11:30 CDT; Hall A, Poster Board: #30 |
| Session: | Gastrointestinal (Colorectal) Cancer |
| Abstract No. & Link: | #3537; abstracts.asco.org/214/AbstView_214_215579.html |
Copies of this poster may be obtained from the American Society of Clinical Oncology.