- Hutchmed
- | Announcements & Press Releases
London: Friday, June 29, 2018: Hutchison China MediTech Limited (“Chi-Med”) (AIM/Nasdaq: HCM) hereby notifies the market that as at June 29, 2018, the issued share capital of Chi-Med consisted of 66,532,683 ordinary shares of US$1.00 each, with each share carrying one right to vote and with no shares held in treasury.
The above figure of 66,532,683 may be used by shareholders as the denominator for the calculations by which they could determine if they are required to notify their interest in, or a change to their interest in, Chi-Med under the Financial Conduct Authority’s Disclosure Rules and Transparency Rules.
For illustrative purposes only, the 66,532,683 ordinary shares would be equivalent to 66,532,683 CREST depositary interests (each equating to one ordinary share) which are traded on AIM or, if the CREST depositary interests were converted in their entirety, equivalent to 133,065,366 American depositary shares (each equating to one-half of one ordinary share) which are traded on Nasdaq.
About Chi-Med
Chi-Med is an innovative biopharmaceutical company which researches, develops, manufactures and sells pharmaceuticals and healthcare products. Its Innovation Platform, Hutchison MediPharma Limited, focuses on discovering and developing innovative therapeutics in oncology and autoimmune diseases for the global market. Its Commercial Platform manufactures, markets, and distributes prescription drugs and consumer health products in China.
Chi-Med is majority owned by the multinational conglomerate CK Hutchison Holdings Limited (SEHK: 1). For more information, please visit: www.chi-med.com.
CONTACTS
Investor Enquiries
Mark Lee, Senior Vice President,
Corporate Finance & Development
+852 2121 8200
U.K. & International Media Enquiries
Anthony Carlisle, Citigate Dewe Rogerson
+44 7973 611 888 (Mobile)
anthony.carlisle@cdrconsultancy.co.uk
U.S. Based Media Enquiries
Brad Miles, Solebury Trout
+1 (917) 570 7340 (Mobile)
bmiles@troutgroup.com
Susan Duffy, Solebury Trout
+1 (917) 499 8887 (Mobile)
sduffy@troutgroup.com
Investor Relations
Xuan Yang, Solebury Trout
+1 (415) 971 9412 (Mobile)
xyang@troutgroup.com
David Dible, Citigate Dewe Rogerson
+44 7967 566 919 (Mobile)
david.dible@citigatedewerogerson.com
Panmure Gordon (UK) Limited
Richard Gray / Andrew Potts
+44 (20) 7886 2500
London: Friday, June 29, 2018: Hutchison China MediTech Limited (“Chi-Med”) (AIM/Nasdaq: HCM) announces the following blocklisting six monthly return:
| 1. | Name of applicant: | Hutchison China MediTech Limited | |
| 2. | Name of scheme: | (a) | Share Option Scheme conditionally adopted by Hutchison China MediTech Limited in 2005 (“2005 HCML Share Option Scheme”) |
| (b) | Share Option Scheme conditionally adopted by Hutchison China MediTech Limited in 2015 (“2015 HCML Share Option Scheme”) | ||
| 3. | Period of return: | From December 29, 2017 to June 28, 2018 | |
| 4. | Balance under scheme from previous return: | (a) | 2005 HCML Share Option Scheme: 304,999 ordinary shares of US$1 each |
| (b) | 2015 HCML Share Option Scheme: 1,000,000 ordinary shares of US$1 each | ||
| 5. | The amount by which the block scheme has been increased, if the scheme has been increased since the date of the last return: | (a) | 2005 HCML Share Option Scheme: Nil |
| (b) | 2015 HCML Share Option Scheme: 1,425,597 ordinary shares of US$1 each | ||
| 6. | Number of securities issued/allotted under scheme during period: | (a) | 2005 HCML Share Option Scheme: 85,646 ordinary shares of US$1 each |
| (b) | 2015 HCML Share Option Scheme: Nil ordinary shares of US$1 each | ||
| 7. | Balance under scheme not yet issued/allotted at end of the period: | (a) | 2005 HCML Share Option Scheme: 219,353 ordinary shares of US$1 each |
| (b) | 2015 HCML Share Option Scheme: 2,425,597 ordinary shares of US$1 each | ||
| 8. | Number and class of securities originally listed and the date of admission: | (i) | 2,560,606 ordinary shares of US$1 each admitted on June 26, 2007 |
| (ii) | 1,000,000 ordinary shares of US$1 each admitted on June 20, 2016 | ||
| (iii) | 1,425,597 ordinary shares of US$1 each admitted on April 27, 2018 | ||
| 9. | Total number of securities in issue at the end of the period: | 66,532,683 ordinary shares of US$1 each | |
| Name of contact: | Christian Hogg | ||
| Address of contact: | 21/F., Hutchison House, 10 Harcourt Road, Hong Kong | ||
| Telephone number of contact: | +852 2121 8200 | ||
About Chi-Med
Chi-Med is an innovative biopharmaceutical company which researches, develops, manufactures and sells pharmaceuticals and healthcare products. Its Innovation Platform, Hutchison MediPharma Limited, focuses on discovering and developing innovative therapeutics in oncology and autoimmune diseases for the global market. Its Commercial Platform manufactures, markets, and distributes prescription drugs and consumer health products in China.
Chi-Med is majority owned by the multinational conglomerate CK Hutchison Holdings Limited (SEHK: 1). For more information, please visit: www.chi-med.com.
CONTACTS
Investor Enquiries
Mark Lee, Senior Vice President,
Corporate Finance & Development
+852 2121 8200
U.K. & International Media Enquiries
Anthony Carlisle, Citigate Dewe Rogerson
+44 7973 611 888 (Mobile)
anthony.carlisle@cdrconsultancy.co.uk
U.S. Based Media Enquiries
Brad Miles, Solebury Trout
+1 (917) 570 7340 (Mobile)
bmiles@troutgroup.com
Susan Duffy, Solebury Trout
+1 (917) 499 8887 (Mobile)
sduffy@troutgroup.com
Investor Relations
Xuan Yang, Solebury Trout
+1 (415) 971 9412 (Mobile)
xyang@troutgroup.com
David Dible, Citigate Dewe Rogerson
+44 7967 566 919 (Mobile)
david.dible@citigatedewerogerson.com
Panmure Gordon (UK) Limited
Richard Gray / Andrew Potts
+44 (20) 7886 2500
London: Friday, June 29, 2018: Hutchison China MediTech Limited (“Chi-Med”) (AIM/Nasdaq: HCM) will be announcing its interim results for the six months ended June 30, 2018 on Friday, July 27, 2018 at 7:00 am British Summer Time (BST).
An analyst presentation will be held at 9:00 am BST (4:00 pm Hong Kong Time) on the same day at Citigate Dewe Rogerson, 3 London Wall Buildings, London, EC2M 5SY, UK, which will be webcast via the company website at www.chi-med.com/investors/event-information. The presentation will be available to download before the analyst presentation begins.
For North America based analysts and investors, Chi-Med will also host a conference call with Q&A at 9:00 am Eastern Daylight Time (2:00 pm BST).
Details of the analyst presentation and conference call dial-in will be provided in the financial results announcement. A replay will also be available on the website shortly after each event.
About Chi-Med
Chi-Med is an innovative biopharmaceutical company which researches, develops, manufactures and sells pharmaceuticals and healthcare products. Its Innovation Platform, Hutchison MediPharma Limited, focuses on discovering and developing innovative therapeutics in oncology and autoimmune diseases for the global market. Its Commercial Platform manufactures, markets, and distributes prescription drugs and consumer health products in China.
Chi-Med is majority owned by the multinational conglomerate CK Hutchison Holdings Limited (SEHK: 1). For more information, please visit: www.chi-med.com.
CONTACTS
Investor Enquiries
Mark Lee, Senior Vice President,
Corporate Finance & Development
+852 2121 8200
U.K. & International Media Enquiries
Anthony Carlisle, Citigate Dewe Rogerson
+44 7973 611 888 (Mobile)
anthony.carlisle@cdrconsultancy.co.uk
U.S. Based Media Enquiries
Brad Miles, Solebury Trout
+1 (917) 570 7340 (Mobile)
bmiles@troutgroup.com
Susan Duffy, Solebury Trout
+1 (917) 499 8887 (Mobile)
sduffy@troutgroup.com
Investor Relations
Xuan Yang, Solebury Trout
+1 (415) 971 9412 (Mobile)
xyang@troutgroup.com
David Dible, Citigate Dewe Rogerson
+44 7967 566 919 (Mobile)
david.dible@citigatedewerogerson.com
Panmure Gordon (UK) Limited
Richard Gray / Andrew Potts
+44 (20) 7886 2500
Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer
The FRESCO Randomized Clinical Trial
 Jin Li; Shukui Qin; Rui-Hua Xu; Lin Shen; Jianming Xu; Yuxian Bai; Lei Yang; Yanhong Deng; Zhen-dong Chen; Haijun Zhong; Hongming Pan; Weijian Guo; Yongqian Shu; Ying Yuan; Jianfeng Zhou; Nong Xu; Tianshu Liu; Dong Ma; Changping Wu; Ying Cheng; Donghui Chen; Wei Li; Sanyuan Sun; Zhuang Yu; Peiguo Cao; Haihui Chen; Jiejun Wang; Shubin Wang; Hongbing Wang; Songhua Fan; Ye Hua; Weiguo Su
Jin Li; Shukui Qin; Rui-Hua Xu; Lin Shen; Jianming Xu; Yuxian Bai; Lei Yang; Yanhong Deng; Zhen-dong Chen; Haijun Zhong; Hongming Pan; Weijian Guo; Yongqian Shu; Ying Yuan; Jianfeng Zhou; Nong Xu; Tianshu Liu; Dong Ma; Changping Wu; Ying Cheng; Donghui Chen; Wei Li; Sanyuan Sun; Zhuang Yu; Peiguo Cao; Haihui Chen; Jiejun Wang; Shubin Wang; Hongbing Wang; Songhua Fan; Ye Hua; Weiguo Su
Abstract
Importance
Patients with metastatic colorectal cancer (CRC) have limited effective and tolerable treatment options.
Objective
To evaluate the efficacy and safety of oral fruquintinib, a vascular endothelial growth factor receptor (VEGFR) inhibitor, as third-line or later therapy in patients with metastatic CRC.
Design, Setting, and Participants
FRESCO (Fruquintinib Efficacy and Safety in 3+ Line Colorectal Cancer Patients) was a randomized, double-blind, placebo-controlled, multicenter (28 hospitals in China), phase 3 clinical trial. From December 2014 to May 2016, screening took place among 519 patients aged 18 to 75 years who had metastatic CRC that progressed after at least 2 lines of chemotherapy but had not received VEGFR inhibitor therapy; 416 met the eligibility criteria and were stratified by prior anti-VEGF therapy and K-ras status. The final date of follow-up was January 17, 2017.
Interventions
Patients were randomized in a 2:1 ratio to receive either fruquintinib, 5 mg (n = 278) or placebo (n = 138) orally, once daily for 21 days, followed by 7 days off in 28-day cycles, until disease progression, intolerable toxicity, or study withdrawal.
Main Outcomes and Measures
The primary end point was overall survival. Key secondary efficacy endpoints were progression-free survival (time from randomization to disease progression or death), objectiveresponse rate (confirmed complete or partial response), and disease control rate (complete or partial response, or stabledisease recorded ≥8 weeks postrandomization). Duration of response was also assessed. Safety outcomes included treatment-emergent adverse events.
Results
Of the 416 randomized patients (mean age, 54.6 years; 161 [38.7%] women), 404 (97.1%) completed the trial. Median overall survival was significantly prolonged with fruquintinib compared with placebo (9.3 months [95% CI, 8.2-10.5] vs 6.6 months [95% CI, 5.9-8.1]); hazard ratio (HR) for death, 0.65 (95% CI, 0.51-0.83; P < .001). Median progression-free survival was also significantly increased with fruquintinib (3.7 months [95% CI, 3.7-4.6] vs 1.8 months [95% CI, 1.8-1.8] months); HR for progression or death, 0.26 (95% CI, 0.21 to 0.34; P < .001). Grades 3 and 4 treatment-emergent adverse events occurred in 61.2% (170) of patients who received fruquintinib and 19.7% (27) who received placebo. Serious adverse events were reported by 15.5% (43) of patients in the fruquintinib group and 5.8% (8) in the placebo group, with 14.4% (40) of fruquintinib-treated and 5.1% (7) of placebo-treated patients requiring hospitalization.
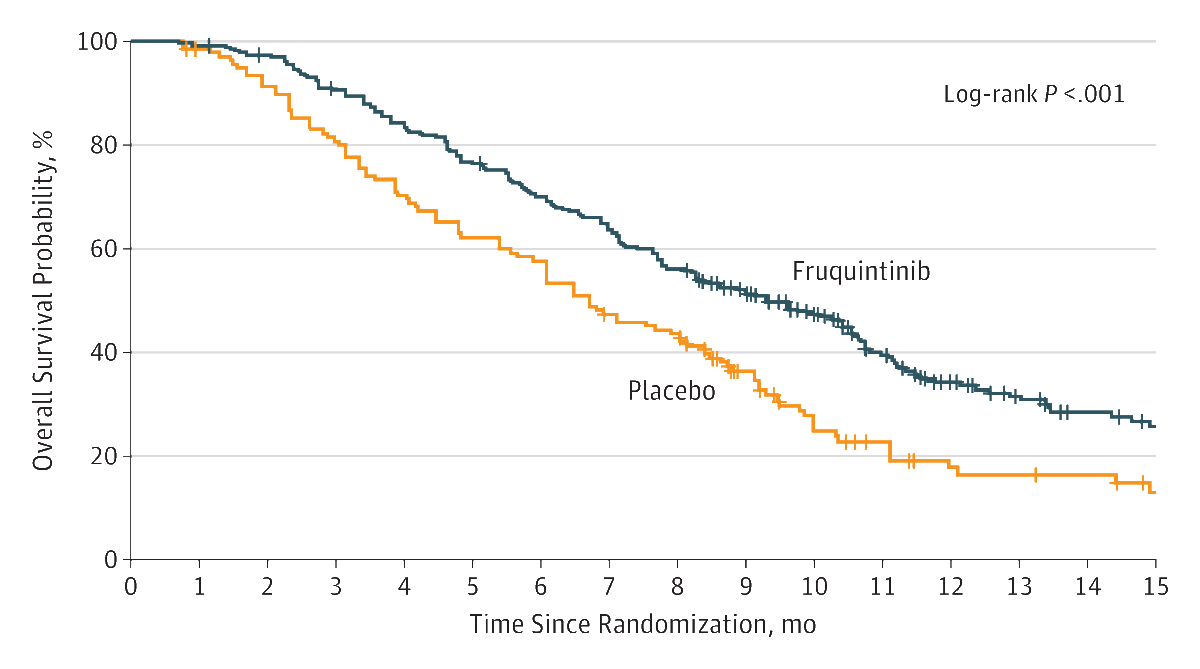
Conclusions and Relevance
Among Chinese patients with metastatic CRC who had tumor progression following at least 2 prior chemotherapy regimens, oral fruquintinib compared with placebo resulted in a statistically significant increase in overall survival. Further research is needed to assess efficacy outside of China.
Trial Registration ClinicalTrials.gov Identifier: NCT02314819
Citations and Links
Please follow the link below to access the publication:
JAMA. 2018;319(24):2486-2496.
London: Thursday, June 7, 2018: Hutchison China MediTech Limited (“Chi-Med”) (AIM/Nasdaq: HCM) announces that on June 6, 2018, it granted share options under the Share Option Scheme conditionally adopted by Chi-Med at its Annual General Meeting in 2015 (the “2015 HCML Share Option Scheme”).
Chi-Med granted 36,936 share options under its 2015 HCML Share Option Scheme to certain employees to subscribe for Ordinary Shares subject to the acceptance of the grantees. Details of such share options granted prescribed are as follows:
Date of grant: June 6, 2018
Exercise price of share options granted: GBP41.66 per Ordinary Share
Number of share options granted: 36,936 (each share option shall entitle the holder thereof to subscribe for one Ordinary Share)
Closing market price of Ordinary Shares on the date of grant: GBP41.40 per Ordinary Share
Validity period of the share options: From June 6, 2018 to June 5, 2028
About Chi-Med
Chi-Med is an innovative biopharmaceutical company which researches, develops, manufactures and sells pharmaceuticals and healthcare products. Its Innovation Platform, Hutchison MediPharma Limited, focuses on discovering and developing innovative therapeutics in oncology and autoimmune diseases for the global market. Its Commercial Platform manufactures, markets, and distributes prescription drugs and consumer health products in China.
Chi-Med is majority owned by the multinational conglomerate CK Hutchison Holdings Limited (SEHK: 1). For more information, please visit: www.chi-med.com.
Forward Looking Statements
This announcement contains forward-looking statements within the meaning of the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements involve risks and uncertainties. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see Chi-Med’s filings with the U.S. Securities and Exchange Commission and on AIM. Chi-Med undertakes no obligation to update or revise the information contained in this announcement, whether as a result of new information, future events or circumstances or otherwise.
CONTACTS
Investor Enquiries
Mark Lee, Senior Vice President,
Corporate Finance & Development
+852 2121 8200
U.K. & International Media Enquiries
Anthony Carlisle, Citigate Dewe Rogerson
+44 7973 611 888 (Mobile)
anthony.carlisle@cdrconsultancy.co.uk
U.S. Based Media Enquiries
Brad Miles, Solebury Trout
+1 (917) 570 7340 (Mobile)
bmiles@troutgroup.com
Susan Duffy, Solebury Trout
+1 (917) 499 8887 (Mobile)
sduffy@troutgroup.com
Investor Relations
Xuan Yang, Solebury Trout
+1 (415) 971 9412 (Mobile)
xyang@troutgroup.com
David Dible, Citigate Dewe Rogerson
+44 7967 566 919 (Mobile)
david.dible@citigatedewerogerson.com
Panmure Gordon (UK) Limited
Richard Gray / Andrew Potts
+44 (20) 7886 2500
Press Release
– Chi-Med makes additional FRESCO presentations at ASCO 2018, following oral presentation at ASCO 2017 showing that study met all endpoints with a manageable safety profile and lower off-target toxicities compared to other targeted therapies –
– Subgroup analysis showed consistent survival benefit across all key subgroups –
– Quality-adjusted survival analysis showed quality-of-life benefits –
London: Monday, June 4, 2018: Hutchison China MediTech Limited (“Chi-Med”) (AIM/Nasdaq: HCM) today announced that further data from the FRESCO Phase III study in 416 patients with locally advanced or metastatic colorectal cancer (“CRC”) were presented at the 2018 American Society of Clinical Oncology (“ASCO”) Annual Meeting, held in Chicago, Illinois from June 1 to 5, 2018.
Fruquintinib is a highly selective and potent oral inhibitor of vascular endothelial growth factor (“VEGF”) receptors 1, 2 and 3. The FRESCO dataset is a part of the New Drug Application (“NDA”) filed and accepted by the China National Drug Administration (the “CNDA”). Additional clinical trials are ongoing in China for lung cancer (the third-line FALUCA Phase III study and the first-line Iressa® combination Phase II study) and gastric cancer (the second-line FRUTIGA Phase III study), as well as in the United States (Phase I bridging study).
The two presentations were as follows:
Subgroup analysis by prior anti-VEGF or anti-EGFR target therapy in FRESCO, a randomized, double-blind, Phase III trial comparing fruquintinib versus placebo plus best supportive care in Chinese patients with metastatic colorectal cancer
| Presenter: | Ruihua Xu |
| Other Authors: | Jin Li, Yu-Xian Bai, Yanhong Deng, Lei Yang, Haijun Zhong, Zhendong Chen, Hongming Pan, Weijian Guo, Yongqian Shu, Ying Yuan, Jianming Xu, Lin Shen, Ning Wang, Xin Wang, Haidong Chi, Jack Peng, Ye Hua, Weiguo Su, Shukui Qin |
| Time & Location: | Sunday, June 3, 08:00 – 11:30 CDT; Hall A, Poster Board: #30 |
| Session: | Gastrointestinal (Colorectal) Cancer |
| Abstract No. & Link: | #3537; abstracts.asco.org/214/AbstView_214_215579.html |
| Poster Link [i]: | 16ealJfSDOCUUwuY6Icm22 |
In FRESCO, fruquintinib demonstrated a statistically significant and clinically meaningful benefit in third-line metastatic CRC patients in China. This analysis explored possible effects of prior target therapy on the efficacy and safety of fruquintinib by analyzing the subgroups of patients with prior target therapy (“PTT”) and those without prior target therapy (“non-PTT”). The results of this analysis showed that fruquintinib had clinically meaningful benefits in third-line metastatic CRC patients regardless of PTT without observed accumulative toxicity.
Results previously presented at the 20th Annual Meeting of the Chinese Society of Clinical Oncology showed that the benefits of fruquintinib were generally consistent across all subgroups. Among a total of 278 fruquintinib-treated patients, 111 received PTT. In the PTT subgroup, fruquintinib significantly prolonged overall survival (“OS”) (Median OS: 7.69 months vs 5.98 months; HR = 0.63; p = 0.023) and progression-free survival (“PFS”) (Median PFS: 3.65 months vs 1.84 months; HR = 0.24; p < 0.001) compared to placebo. Patients who received prior anti-VEGF treatment (N = 84) also benefited from fruquintinib in OS (Median 7.20 months vs 5.91 months; HR = 0.68; p=0.066) and PFS (Median 3.48 months vs 1.84 months; HR = 0.24; p < 0.001). In the non-PTT subgroup, the median OS was 10.35 months for fruquintinib vs 6.93 months for placebo (HR = 0.63; p = 0.01), and the median PFS for fruquintinib was 3.81 months vs 1.84 months for placebo (HR = 0.28; p < 0.001).
Additional data presented at this year’s ASCO showed that there were no observed accumulative Grade ≥3 treatment-emergent adverse events in the PTT subgroup. The Grade ≥3 treatment-emergent adverse events rates of fruquintinib were similar in PTT and non-PTT subgroup (61.3% and 61.1%). This subgroup analysis result is consistent with the previously reported FRESCO intent-to-treatment population result.
Quality-adjusted time without symptoms or toxicity (Q-TWiST) of patients with metastatic colorectal cancer treated with fruquintinib in the randomized Phase III FRESCO trial
| Presenter: | Yu-Xian Bai |
| Other Authors: | Hongyan Li, Ning Wang, Xiaojun Guo, Wei Wang, Songhua Fan, Jian-Ming Xu, Lin Shen |
| Time & Location: | Sunday, June 3, 08:00 – 11:30 CDT; Hall A, Poster Board: #37 |
| Session: | Gastrointestinal (Colorectal) Cancer |
| Abstract No. & Link: | #3544; abstracts.asco.org/214/AbstView_214_224293.html |
| Poster Link [ii]: | 4l9OppVsv6UKE20CM4uQYy |
This ad-hoc analysis aimed to compare the quality-adjusted survival between the two arms of the FRESCO study using quality-adjusted time without symptoms or toxicity (“Q-TWiST”) methodology and to investigate the Q-TWiST benefit of fruquintinib treatment among subgroups. Q-TWiST is a tool to evaluate relative clinical benefit-risk from patient’s perspective and has been widely used in oncology treatment assessment. The survival time for each patient was divided into 3 portions: TOX (time with ≥ Grade 3 toxicity before progression), TWiST (time without symptoms or ≥ Grade 3 toxicity), and REL (time from progression or relapse until death or end of follow-up).
Patients treated with fruquintinib had longer Q-TWiST periods compared to patients treated with placebo. Q-TWiST benefits were observed regardless of prior lines of chemotherapy and target treatment with anti-VEGF or anti-EGFR. The relative improvement of Q-TWiST with fruquintinib represents a clinically important quality-of-life benefit for metastatic CRC patients.
Further information about the ASCO annual meeting is available at am.asco.org.
About Fruquintinib
Fruquintinib (HMPL-013) is a highly selective small molecule drug candidate that has been shown to inhibit VEGF receptors 24 hours a day via an oral dose, with lower off-target toxicities compared to other targeted therapies. Its tolerability, along with its clean drug-drug interaction profile demonstrated to date, may enable rational combination with other cancer therapies such as in our ongoing clinical trials of fruquintinib in combination with chemotherapy and targeted therapy. VEGF receptors play a pivotal role in tumor-related angiogenesis, and inhibition of VEGFR represents an important therapeutic strategy in blocking the development of new blood vessels essential for tumors to grow and invade.
About Fruquintinib in CRC in China
The CNDA, formerly the China Food and Drug Administration, acknowledged acceptance of the NDA for fruquintinib for the treatment of patients with advanced CRC in June 2017. Fruquintinib was subsequently awarded priority review status in view of its significant clinical value, according to a CNDA announcement in September 2017. The NDA is supported by data from the successful FRESCO study, which was highlighted in an oral presentation at the ASCO Annual Meeting held on June 5, 2017. Additional details about this study can be found at clinicaltrials.gov, using identifier NCT02314819. The FRESCO study followed an initial Phase I trial in 40 solid tumor patients, a Phase Ib study in 62 CRC patients, and a Phase II clinical trial in 71 CRC patients.
Other Fruquintinib Development Programs
Lung cancer in China: Fruquintinib is being studied in China in a Phase III registration study, known as FALUCA, in non-small cell lung cancer (“NSCLC”) patients. FALUCA is a randomized, double-blind, placebo-controlled, multi-center study of fruquintinib targeted at treating patients with advanced non-squamous NSCLC who have failed two lines of systemic chemotherapy. The trial completed enrollment of 527 patients in February 2018 (clinicaltrials.gov identifier NCT02691299). It was initiated following a similar Phase II clinical trial in 91 third-line NSCLC patients. Results of the Phase II study were highlighted in an oral presentation at the 17th World Conference on Lung Cancer on December 6, 2016 (clinicaltrials.gov identifier NCT02590965).
Along with FALUCA, fruquintinib is concurrently being studied in a Phase II study in combination with Iressa® (gefitinib) in the first-line setting for patients with advanced or metastatic NSCLC (clinicaltrials.gov identifier NCT02976116). Preliminary results were highlighted in an oral presentation at the 18th World Conference on Lung Cancer on October 16, 2017.
Gastric cancer in China: In October 2017, Chi-Med initiated a pivotal Phase III clinical trial of fruquintinib in combination with Taxol® (paclitaxel), known as the FRUTIGA study, for the treatment of patients with advanced gastric or gastroesophageal junction (“GEJ”) adenocarcinoma. The FRUTIGA study is a randomized, double-blind, placebo-controlled, multi-center trial expected to enroll over 500 gastric or GEJ adenocarcinoma patients who have progressed after first-line standard chemotherapy (clinicaltrials.gov identifier NCT03223376). The FRUTIGA study follows a Phase Ib/II clinical trial that demonstrated that combination therapy of fruquintinib and Taxol® in such patients was generally well-tolerated with promising tumor response (clinicaltrials.gov identifier NCT02415023).
In China, fruquintinib is jointly developed with Eli Lilly and Company.
United States bridging trial: In December 2017, Chi-Med initiated a multi-center, open-label, Phase I clinical study to evaluate the safety, tolerability and pharmacokinetics of fruquintinib in U.S. patients with advanced solid tumors (clinicaltrials.gov identifier NCT03251378).
About Chi-Med
Chi-Med is an innovative biopharmaceutical company which researches, develops, manufactures and sells pharmaceuticals and healthcare products. Its Innovation Platform, Hutchison MediPharma Limited, focuses on discovering and developing innovative therapeutics in oncology and autoimmune diseases for the global market. Its Commercial Platform manufactures, markets, and distributes prescription drugs and consumer health products in China.
Chi-Med is majority owned by the multinational conglomerate CK Hutchison Holdings Limited (SEHK: 1). For more information, please visit: www.chi-med.com.
Iressa® is a trademark of the AstraZeneca PLC group of companies. Taxol® is a trademark of The Bristol-Myers Squibb Company group of companies.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect Chi-Med’s current expectations regarding future events, including its expectations for the clinical development of fruquintinib, plans to initiate clinical studies for fruquintinib, its expectations as to whether such studies would meet their primary or secondary endpoints, and its expectations as to the timing of the completion and the release of results from such studies. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding enrollment rates, timing and availability of subjects meeting a study’s inclusion and exclusion criteria, changes to clinical protocols or regulatory requirements, unexpected adverse events or safety issues, the ability of fruquintinib to meet the primary or secondary endpoint of a study, to obtain regulatory approval in different jurisdictions, to gain commercial acceptance after obtaining regulatory approval, the potential market of fruquintinib for a targeted indication and the sufficiency of funding. In addition, as certain studies rely on the use of Iressa® (gefitinib) or Taxol® (paclitaxel) as combination therapeutics with fruquintinib, such risks and uncertainties include assumptions regarding the safety, efficacy, supply and continued regulatory approval of Iressa® and Taxol®. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see Chi-Med’s filings with the U.S. Securities and Exchange Commission and on AIM. Chi-Med undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
CONTACTS
Investor Enquiries
Mark Lee, Senior Vice President,
Corporate Finance & Development
+852 2121 8200
U.K. & International Media Enquiries
Anthony Carlisle, Citigate Dewe Rogerson
+44 7973 611 888 (Mobile)
anthony.carlisle@cdrconsultancy.co.uk
U.S. Based Media Enquiries
Brad Miles, Solebury Trout
+1 (917) 570 7340 (Mobile)
bmiles@troutgroup.com
Susan Duffy, Solebury Trout
+1 (917) 499 8887 (Mobile)
sduffy@troutgroup.com
Investor Relations
Xuan Yang, Solebury Trout
+1 (415) 971 9412 (Mobile)
xyang@troutgroup.com
David Dible, Citigate Dewe Rogerson
+44 7967 566 919 (Mobile)
david.dible@citigatedewerogerson.com
Panmure Gordon (UK) Limited
Richard Gray / Andrew Potts
+44 (20) 7886 2500
[i] Copies of this poster obtained through this hyperlink are for personal use only and may not be reproduced without permission from the American Society of Clinical Oncology and the author of the poster: https://mdda-mobiledocdelivery.com/Download/16ealJfSDOCUUwuY6Icm22
[ii] Copies of this poster obtained through this hyperlink are for personal use only and may not be reproduced without permission from American Society of Clinical Oncology and the author of the poster: https://mdda-mobiledocdelivery.com/Download/4l9OppVsv6UKE20CM4uQYy
Quality-adjusted time without symptoms or toxicity (Q-TWiST) of patients with metastatic colorectal cancer treated with fruquintinib in FRESCO
| Presenter: | Yu-Xian Bai |
| Other Authors: | Hongyan Li, Ning Wang, Xiaojun Guo, Wei Wang, Songhua Fan, Jian-Ming Xu, Lin Shen |
| Time & Location: | Sunday, June 3, 08:00 – 11:30 CDT; Hall A, Poster Board: #37 |
| Session: | Gastrointestinal (Colorectal) Cancer |
| Abstract No. & Link: | #3544; abstracts.asco.org/214/AbstView_214_224293.html |
Copies of this poster can be obtained from American Society of Clinical Oncology and the author of the poster.
| Presenter: | Ruihua Xu |
| Other Authors: | Jin Li, Yu-Xian Bai, Yanhong Deng, Lei Yang, Haijun Zhong, Zhendong Chen, Hongming Pan, Weijian Guo, Yongqian Shu, Ying Yuan, Jianming Xu, Lin Shen, Ning Wang, Xin Wang, Haidong Chi, Jack Peng, Ye Hua, Weiguo Su, Shukui Qin |
| Time & Location: | Sunday, June 3, 08:00 – 11:30 CDT; Hall A, Poster Board: #30 |
| Session: | Gastrointestinal (Colorectal) Cancer |
| Abstract No. & Link: | #3537; abstracts.asco.org/214/AbstView_214_215579.html |
Copies of this poster may be obtained from the American Society of Clinical Oncology.