- Hutchmed
- | Announcements & Press Releases
— Trial met primary endpoint of overall survival and all secondary endpoints —
— Overall safety consistent with fruquintinib known profile —
— Plans for regulatory submissions underway in the U.S., Europe and Japan —
— Results to be submitted to an upcoming medical meeting —
Hong Kong, Shanghai & Florham Park, NJ — Monday, August 8, 2022: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM: HCM, HKEX: 13) today announces that the pivotal global Phase 3 FRESCO-2 trial evaluating the investigational use of fruquintinib met its primary endpoint of overall survival (“OS”) in patients with advanced, refractory metastatic colorectal cancer (“CRC”).
The FRESCO-2 study was a multi-regional clinical trial conducted in the U.S., Europe, Japan and Australia that investigated fruquintinib plus best supportive care (“BSC”) vs placebo plus BSC in patients with metastatic CRC who had progressed on standard chemotherapy and relevant biologic agents and who had progressed on, or were intolerant to, TAS-102 and/or regorafenib. In addition to OS, a statistically-significant improvement in progression-free survival (“PFS”), a key secondary endpoint, was observed. The safety profile of fruquintinib in FRESCO-2 was consistent with previously reported studies. Full results will be submitted for presentation at an upcoming medical meeting.
HUTCHMED has been in communication with regulatory agencies globally regarding the FRESCO-2 trial design and conduct and will discuss these data with the agencies in the U.S., Europe and Japan with the intent to submit marketing authorization applications as soon as possible. The U.S. FDA granted Fast Track Designation for the development of fruquintinib for the treatment of patients with metastatic CRC in June 2020.
“We are very happy to see the positive outcomes of the FRESCO-2 study which offers a potential new treatment for patients with advanced metastatic colorectal cancer, where the unmet need is very high and patients have limited treatment options,” said Dr Marek Kania, Executive Vice President, Managing Director and Chief Medical Officer of HUTCHMED International. “Results from the global FRESCO-2 study supplement findings from the original FRESCO study that led to the marketing approval and commercialization of fruquintinib in China. We would like to thank the patients, their families, and the healthcare professionals who participated in this study and helped achieve this important milestone.”
Professor Cathy Eng, MD, FACP, FASCO, David H. Johnson Endowed Chair in Surgical and Medical Oncology and Co-Leader, Gastrointestinal Cancer Research Program, at the Vanderbilt-Ingram Cancer Center, who served as the FRESCO2 co-PI and Steering Committee member said: “Completion of the international FRESCO-2 phase III trial in a timely fashion during the era of COVID-19 isolation demonstrates the unmet need for new therapeutic agents in metastatic colorectal cancer. By meeting the primary endpoint of OS with a secondary endpoint of PFS, fruquintinib provides a significant potential new option for our refractory colorectal cancer patients. As an oral agent, fruquintinib also provides added convenience for our patients. Based on fruquintinib’s profile, we will likely see further exploration in future clinical trials in different settings. This is extremely encouraging, and I look forward to seeing the final results.”
Dr Weiguo Su, Chief Executive Officer and Chief Scientific Officer of HUTCHMED, said: “We are pleased to have successfully completed our first multi-regional clinical trial, FRESCO-2, to support the global registration of fruquintinib. It has already benefited patients with advanced CRC in China since its launch in 2018. It is also being evaluated alone and in combination with other agents in various tumor types in ongoing studies around the world.”
HUTCHMED retains all commercial rights to fruquintinib outside of China. In China, where fruquintinib is marketed under the brand name ELUNATE®, HUTCHMED is partnered with Eli Lilly and Company and is responsible for development and execution of all on-the-ground medical detailing, promotion and local and regional marketing. Fruquintinib is not approved for use outside of China.
About CRC
CRC is a cancer that starts in either the colon or rectum. CRC is the third most common cancer worldwide, estimated to have caused more than 915,000 deaths in 2020.[i] In the U.S., an estimated 151,000 people will have been diagnosed with CRC and 53,000 people will have died from CRC in 2022.[ii] In Europe, CRC is the second most common cancer, with an estimated 507,000 new cases and 240,000 deaths in 2020.1 In Japan, CRC is the most common cancer, with an estimated 147,000 new cases and 59,000 deaths in 2020.1
About Fruquintinib
Fruquintinib is a highly selective and potent oral inhibitor of VEGFR-1, -2 and -3. VEGFR inhibitors play a pivotal role in blocking tumor angiogenesis. Fruquintinib was designed to improve kinase selectivity to minimize off-target toxicities, improve tolerability and provide more consistent target coverage. The generally good tolerability in patients to date, along with fruquintinib’s low potential for drug-drug interaction based on preclinical assessment, suggests that it may also be highly suitable for combinations with other anti-cancer therapies.
About Fruquintinib Approval in China
Metastatic CRC in China: Fruquintinib was approved for marketing by the China National Medical Products Administration (NMPA) in September 2018 and commercially launched in China in late November 2018 under the brand name ELUNATE®. It has been included in the China National Reimbursement Drug List (NRDL) since January 2020. ELUNATE® is indicated for the treatment of patients with metastatic CRC who have been previously treated with fluoropyrimidine, oxaliplatin and irinotecan, including those who have previously received anti-VEGF therapy and/or anti-EGFR therapy (RAS wild type). Results of the FRESCO study[iii], a Phase III pivotal registration trial of fruquintinib in 416 patients with metastatic CRC in China, were published in The Journal of the American Medical Association, JAMA, in June 2018 (clinicaltrials.gov identifier: NCT02314819).
About Fruquintinib Development Beyond CRC Monotherapy
The safety and efficacy of fruquintinib for the following investigational uses have not been established and there is no guarantee that it will receive health authority approval or become commercially available in any country for the uses being investigated:
Gastric Cancer (“GC”) in China: The FRUTIGA study is a randomized, double-blind, Phase III trial evaluating the efficacy and safety of fruquintinib combined with paclitaxel for the treatment of patients with advanced gastric or esophagogastric junction (“GEJ”) adenocarcinoma who did not respond to first-line standard chemotherapy. Approximately 700 patients have received either fruquintinib combined with paclitaxel or placebo combined with paclitaxel. The co-primary efficacy endpoints are OS and PFS (clinicaltrials.gov identifier: NCT03223376).
Immunotherapy combinations: HUTCHMED has entered into collaboration agreements to evaluate the safety, tolerability and efficacy of fruquintinib in combination with PD-1 monoclonal antibodies, including with tislelizumab (BGB-A317, developed by BeiGene, Ltd) and sintilimab (IBI308, developed by Innovent Biologics, Inc. and marketed as TYVYT® in China).
- Metastatic breast, endometrial, and colorectal cancers in the U.S.: HUTCHMED initiated this open-label, multi-center, non-randomized, Phase Ib/II study in the U.S. to investigate if the addition of fruquintinib can potentially induce activity to immune checkpoint inhibitor therapy in advanced, refractory triple negative breast cancer (“TNBC”), endometrial cancer, and CRC. Additional details of the study may be found at clinicaltrials.gov, using identifier NCT04577963. Safety and preliminary efficacy of fruquintinib as a single agent were demonstrated in advanced solid tumors, including TNBC, in a Phase I study conducted in China (NCT01645215) and a Phase I/Ib study is ongoing in the U.S. (NCT03251378).
- Gastric, colorectal and non-small cell lung cancers in China & Korea: BeiGene, Ltd. initiated this open-label, multi-center, Phase II study to assess the safety and efficacy of fruquintinib in combination with tislelizumab in patients with advanced or metastatic, unresectable GC, CRC or non-small cell lung cancer (“NSCLC”). Additional details of the study may be found at clinicaltrials.gov, using identifier NCT04716634.
- Endometrial cancer and other solid tumors in China: HUTCHMED initiated this open-label, multi-center, non-randomized, Phase II study to assess the safety and efficacy of fruquintinib in combination with sintilimab in patients with advanced cervical cancer, endometrial cancer, GC, hepatocellular carcinoma (HCC), NSCLC or renal cell carcinoma (RCC). Preliminary results of certain cohorts were presented at the 2021 American Society of Clinical Oncology Annual Meeting (ASCO) and the Chinese Society of Clinical Oncology Annual Meeting (CSCO). Following encouraging data in the advanced endometrial cancer cohort, it has been expanded into a single-arm registrational Phase II study of over 130 patients. Additional details of the study may be found at clinicaltrials.gov, using identifier NCT03903705.
About HUTCHMED
HUTCHMED (Nasdaq/AIM: HCM; HKEX: 13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. It has more than 4,900 personnel across all its companies, at the center of which is a team of about 1,800 in oncology/immunology. Since inception it has advanced 13 cancer drug candidates from in-house discovery into clinical studies around the world, with its first three oncology drugs now approved and marketed in China. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This announcement contains forward-looking statements within the meaning of the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the therapeutic potential of fruquintinib for the treatment of patients with advanced CRC and the further clinical development of fruquintinib in this and other indications. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding the timing and outcome of clinical studies and the sufficiency of clinical data to support NDA approval of fruquintinib for the treatment of patients with advanced CRC or other indications in the U.S., Europe, Japan, Australia or other jurisdictions, its potential to gain approvals from regulatory authorities on an expedited basis or at all, the safety profile of fruquintinib, HUTCHMED’s ability to fund, implement and complete its further clinical development and commercialization plans for fruquintinib, the timing of these events, and the impact of the COVID-19 pandemic on general economic, regulatory and political conditions. In addition, as certain studies rely on the use of other drug products such as paclitaxel, tislelizumab and sintilimab as combination therapeutics with fruquintinib, such risks and uncertainties include assumptions regarding the safety, efficacy, supply and continued regulatory approval of these therapeutics. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the U.S. Securities and Exchange Commission, on AIM and on The Stock Exchange of Hong Kong Limited. HUTCHMED undertakes no obligation to update or revise the information contained in this announcement, whether as a result of new information, future events or circumstances or otherwise.
Inside Information
This announcement contains inside information for the purposes of Article 7 of Regulation (EU) No 596/2014 (as it forms part of retained EU law as defined in the European Union (Withdrawal) Act 2018).
[i] The Global Cancer Observatory. Accessed September 21, 2021.
[ii] SEER. Cancer Stat Facts: Colorectal Cancer. National Cancer Institute. https://seer.cancer.gov/statfacts/html/colorect.html. Accessed June 27, 2022.
[iii] Li J, Qin S, Xu RH, et al. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA. 2018;319(24):2486-2496. doi:10.1001/jama.2018.7855.
Today, we begin consolidating the two corporate identities that we have used since our inception. Hutchison China MediTech, or Chi-Med, has been used as our group identity, while Hutchison MediPharma has been the identity of our novel drug R&D operations, under which our oncology products are developed and are now being marketed.
As our pipeline of novel, highly selective cancer drugs gain approval and advance to the international stage, we feel it is important to now have a single and ubiquitous corporate identity, that logically captures the history and brand equity we have built over the past twenty years. Therefore, we have chosen to rename ourselves HUTCHMED.
HUTCHMED is a natural combination of the resonant components from our two separate identities. Our new brand retains our unique essence, whilst signaling a new chapter – one brand that helps patients around the world.
We will gradually change to this name at all levels in the company during 2021.
Our vision remains unaltered: to lead innovation in oncology and immunology drugs, meet unmet medical needs, and become a global biopharmaceutical company.
— First randomized Phase III trial confirming the efficacy of MET inhibition in patients with advanced NSCLC and acquired MET amplification after progression on prior EGFR-TKI treatment —
— Savolitinib and osimertinib combination approved in China in June 2025 —
Hong Kong, Shanghai & Florham Park, NJ — Wednesday, January 14, 2026: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today highlights that results from the SACHI Phase III trial were published in The Lancet. SACHI is a Phase III study of the savolitinib (ORPATHYS®) and osimertinib (TAGRISSO®) combination for the treatment of patients with locally advanced or metastatic epidermal growth factor receptor (“EGFR”) mutation-positive non-small cell lung cancer (“NSCLC”) with MET amplification after disease progression on first-line EGFR tyrosine kinase inhibitor (“TKI”) therapy.
Savolitinib is an oral, potent and highly selective MET TKI being jointly developed by AstraZeneca and HUTCHMED and commercialized by AstraZeneca. Osimertinib is a third-generation, irreversible EGFR TKI. Based on interim data from SACHI, the savolitinib and osimertinib combination was granted regulatory approval in China in June 2025.
“The SACHI trial, now published in The Lancet, provides compelling evidence that savolitinib combined with osimertinib can transform outcomes for patients with EGFR-mutated NSCLC with MET amplification. These findings highlight the combination’s ability to address MET amplification, a critical resistance mechanism, offering clinically meaningful improvements for this challenging patient population,” said Professor Shun Lu, Chief of the Shanghai Lung Cancer Center at Shanghai Chest Hospital, School of Medicine, Shanghai Jiaotong University, and co-leading Principal Investigator of the SACHI trial. “We are particularly encouraged by the consistent benefits observed in patients previously treated with third-generation EGFR-TKIs, where savolitinib plus osimertinib offers a continued all-oral regimen, providing a convenient and well-tolerated solution for this underserved population.” Professor Jie Wang of Cancer Hospital, Chinese Academy of Medical Sciences also served as co-leading Principal Investigator of the SACHI trial.
About the SACHI Phase III Trial
In January 2025, the Independent Data Monitoring Committee (IDMC) of SACHI considered that the study had met the pre-defined primary endpoint of progression-free survival (“PFS”) in a planned interim analysis and, as a result, enrollment into the study has concluded. As of the interim analysis data cut-off of August 30, 2024, a total of 211 patients were randomized to receive the savolitinib and osimertinib combination (n=106) or chemotherapy (n=105). In the intention-to-treat (“ITT”) population, the median PFS assessed by investigator was 8.2 months (95% confidence interval [“CI”] 6.9–11.2) with savolitinib plus osimertinib, compared to 4.5 months (95% CI 3.0–5.4) with chemotherapy (hazard ratio [“HR”] 0.34; 95% CI 0.23–0.49; p<0.0001). The independent review committee (“IRC”) assessed median PFS was 7.2 vs 4.2 months, respectively (HR 0.40; 95% CI 0.28–0.59; p<0.0001).
The investigator-assessed objective response rate (“ORR”) was 58% in the savolitinib plus osimertinib arm compared to 34% for patients in the chemotherapy arm. The disease control rate (DCR) was 89% vs 67%, and the median duration of response (DoR) was 8.4 vs 3.2 months, respectively. The median time to response (TTR) was similar between two arms (1.4 vs 1.5 months). Overall survival (“OS”) data were still evolving and not mature at the time of the interim analysis, with only 37% and 43% OS maturity. At median OS follow-up duration of 17.7 months in the ITT population, savolitinib plus osimertinib arm reported median OS of 22.9 months vs 17.7 months in the chemotherapy arm (HR 0.84). 55 (52%) patients in the chemotherapy arm received subsequent MET inhibitor therapy after disease progression, with 45 (43%) crossing over to savolitinib-osimertinib and ten (10%) subsequently received MET inhibitor. In sensitivity analyses of OS to adjust for this crossover, the OS benefits of the savolitinib plus osimertinib arm were more significant, with HRs ranging from 0.24 to 0.62.
In the third-generation EGFR TKI–naïve subgroup population (i.e. patients previously treated with a first- or second-generation EGFR TKI), investigator-assessed median PFS was 9.8 vs 5.4 months (HR 0.34; 95% CI 0.21–0.56; p<0.0001). Efficacy outcomes in the third-generation EGFR-TKI–treated subgroup were comparable with those in the ITT population. In this subgroup, the investigator-assessed median PFS was 6.9 vs 3.0 months (HR 0.32; 95% CI 0.18–0.57; p<0.0001), and IRC-assessed median PFS was 6.9 vs 3.0 months (HR 0.32; 95% CI 0.18–0.58; p<0.0001).
The safety profile of the savolitinib and osimertinib combination was tolerable and no new safety signals were observed. Treatment-emergent adverse events (“TEAEs”) of Grade 3 or above occurred in 57% of patients in the savolitinib plus osimertinib arm compared to 57% (55 of 96) for patients in the chemotherapy arm. Common Grade ≥3 TEAEs (≥10% in either arm) included decreased neutrophil count (14% vs 26%), decreased white blood cell count (7% vs 13%), and anemia (4% vs 23%).
About NSCLC and MET aberrations
Lung cancer is the leading cause of cancer death, accounting for about one-fifth of all cancer deaths.[1] Lung cancer is broadly split into NSCLC and small cell lung cancer, with 80-85% classified as NSCLC.[2] The majority of NSCLC patients (approximately 75%) are diagnosed with advanced disease, and approximately 10-15% of NSCLC patients in the US and Europe and up to 40-50% of patients in Asia have EGFR-mutated (“EGFRm”) NSCLC.[3],[4],[5],[6],[7]
MET is a tyrosine kinase receptor that has an essential role in normal cell development. MET overexpression and/or amplification can lead to tumor growth and the metastatic progression of cancer cells, and is one of the mechanisms of de novo or acquired resistance to EGFR TKI for metastatic EGFRm NSCLC.[8],[9]
About ORPATHYS®
ORPATHYS® (savolitinib) is an oral, potent and highly selective MET TKI that has demonstrated clinical activity in advanced solid tumors. It blocks atypical activation of the MET receptor tyrosine kinase pathway that occurs because of mutations (such as exon 14 skipping alterations or other point mutations), gene amplification or protein overexpression.
ORPATHYS® is approved in China and is marketed by AstraZeneca for the treatment of adult patients with locally advanced or metastatic NSCLC with MET exon 14 skipping alteration, representing the first selective MET inhibitor approved in China. ORPATHYS® is also approved in China for the treatment of patients with locally advanced or metastatic EGFRm-positive non-squamous NSCLC with MET amplification after disease progression on EGFR TKI therapy, in combination with TAGRISSO®.
It is currently under clinical development for multiple tumor types, including lung, kidney, and gastric cancers as a single treatment and in combination with other medicines.
About TAGRISSO®
TAGRISSO® (osimertinib) is a third-generation, irreversible EGFR-TKI with proven clinical activity in NSCLC, including against central nervous system (CNS) metastases. TAGRISSO® (40mg and 80mg once-daily oral tablets) has been used to treat more than one million patients across its indications worldwide and AstraZeneca continues to explore TAGRISSO® as a treatment for patients across multiple stages of EGFRm NSCLC.
There is an extensive body of evidence supporting the use of TAGRISSO® in EGFRm NSCLC, and it is the only targeted therapy shown to improve patient outcomes across all stages of the disease.
In late-stage disease, TAGRISSO® demonstrated improved outcomes as monotherapy in the FLAURA Phase III trial and in combination with chemotherapy in the FLAURA2 Phase III trial. TAGRISSO® is also being investigated in this setting in combination with ORPATHYS® (savolitinib) in the SAFFRON Phase III trial and in combination with DATROWAY® (datopotamab deruxtecan or Dato-DXd) in the TROPION-Lung14 and TROPION-Lung15 Phase III trials.
TAGRISSO® also showed improved outcomes in early-stage disease in the NeoADAURA and ADAURA Phase III trials and in locally advanced stages in the LAURA Phase III trial. As part of AstraZeneca’s ongoing commitment to treating patients as early as possible in lung cancer, TAGRISSO® is also being investigated in the early-stage adjuvant resectable setting in the ADAURA2 Phase III trial.
About ORPATHYS® and TAGRISSO® Combination Development in EGFR-mutated NSCLC
This combination represents a promising chemotherapy-free oral treatment strategy to address mechanisms of resistance in this setting. Among patients who experience disease progression following treatment with a third-generation EGFR TKI, approximately 15-50% present with MET aberration, depending on the sample type, detection method and assay cut-off used. TAGRISSO® is a third-generation, irreversible EGFR-TKI with proven clinical activity in NSCLC, including against central nervous system metastases. Treatment with ORPATHYS® in combination with TAGRISSO® has been studied extensively in these patients in the TATTON study (NCT02143466) and the SAVANNAH single-arm Phase II study (NCT03778229). Strong data from SAVANNAH presented at the 2025 European Lung Cancer Congress (ELCC) demonstrated high, clinically meaningful and durable ORR, with consistent safety results. The encouraging results led to the initiation of several randomized Phase III trials in this setting including the SACHI trial in China (NCT05015608) and the global SAFFRON trial (NCT05261399), as well as the SANOVO trial in China (NCT05009836).
SACHI: This Phase III trial in China evaluated the combination of ORPATHYS® and TAGRISSO® compared to platinum-based doublet chemotherapy for the treatment of patients with EGFRm, MET-amplified locally advanced or metastatic NSCLC following progression on treatment with an EGFR TKI. Results were presented at the 2025 ASCO Annual Meeting. The treatment combination received approval in China in June 2025.
SAFFRON: This ongoing global Phase III trial is to evaluate the combination of ORPATHYS® and TAGRISSO® compared to platinum-based doublet chemotherapy in patients with EGFRm, MET-overexpressed and/or amplified, locally advanced or metastatic NSCLC following progression on treatment with TAGRISSO®. This received Fast Track Designation from the US FDA and enrollment was completed in October 2025. We look forward to completing this trial to support potential US and other global registration filings.
SANOVO: This ongoing Phase III trial in China is to evaluate the combination of ORPATHYS® and TAGRISSO® compared to TAGRISSO® monotherapy in previously untreated patients with locally advanced or metastatic NSCLC with EGFRm and MET overexpression. Enrollment was completed in August 2025.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the therapeutic potential of ORPATHYS®, the further clinical development for ORPATHYS®, its expectations as to whether any studies on ORPATHYS® would meet their primary or secondary endpoints, and its expectations as to the timing of the completion and the release of results from such studies. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding enrollment rates and the timing and availability of subjects meeting a study’s inclusion and exclusion criteria; changes to clinical protocols or regulatory requirements; unexpected adverse events or safety issues; the ability of ORPATHYS®, including as a combination therapy, to meet the primary or secondary endpoint of a study, to obtain regulatory approval in other jurisdictions and to gain commercial acceptance after obtaining regulatory approval; the potential market of ORPATHYS® for a targeted indication; and HUTCHMED and/or its partner’s ability to fund, implement and complete its further clinical development and commercialization plans for ORPATHYS®, and the timing of these events. In addition, as certain studies rely on the use of other drug products such as TAGRISSO® as combination therapeutics with ORPATHYS®, such risks and uncertainties include assumptions regarding the safety, efficacy, supply and continued regulatory approval of these therapeutics. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
References
[1] World Health Organization. International Agency for Research on Cancer. All cancers fact sheet. Available at: https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf. Accessed November 2022.
[2] American Cancer Society. What is Lung Cancer? Available at: https://www.cancer.org/cancer/lung-cancer/about/what-is.html. Accessed November 2022.
[3] Knight SB, et al. Progress and prospects of early detection in lung cancer. Open Biol. 2017;7(9): 170070.
[4] Keedy VL, et al. American Society of Clinical Oncology Provisional Clinical Opinion: Epidermal Growth Factor Receptor (EGFR) Mutation Testing for Patients with Advanced Non-Small-Cell Lung Cancer Considering First-Line EGFR Tyrosine Kinase Inhibitor Therapy. J Clin Oncol. 2011:29;2121-27.
[5] Zhang Y, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7(48).
[6] Szumera-Ciećkiewicz A, et al. EGFR Mutation Testing on Cytological and Histological Samples in 11. Non-Small Cell Lung Cancer: a Polish, Single Institution Study and Systematic Review of European Incidence. Int J Clin Exp Pathol. 2013:6;2800-12.
[7] Gou LY, et al. Prevalence of driver mutations in non-small-cell lung cancers in the People’s Republic of China. Lung Cancer: Targets and Therapy. 2014; 5; 1–9.
[8] Uchikawa E, et al. Structural basis of the activation of c-MET receptor. Nat Commun. 2021;12(4074).
[9] Wang Q, et al. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. Journal of Hematology & Oncology. 2019;63.
Download latest HUTCHMED corporate presentation
— Delivers rapid, durable responses in wAIHA, the more common form of this potentially life-threatening disease —
Hong Kong, Shanghai & Florham Park, NJ — Wednesday, January 7, 2026: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that the Phase III registration part of the ESLIM-02 clinical trial of sovleplenib, a novel spleen tyrosine kinase (“Syk”) inhibitor, in adult patients with warm antibody autoimmune hemolytic anemia (“wAIHA”) in China has met its primary endpoint of durable hemoglobin (Hb) response rate within weeks 5 to 24 of treatment.
Autoimmune hemolytic anemia (“AIHA”) is an autoimmune disorder characterized by the destruction of red blood cells (“RBCs”) due to the production of antibodies against RBC. The incidence of AIHA is estimated to be 0.8-3.0/100,000 adults per year with an estimated prevalence of 17 per 100,000 adults and a death rate of 8‑11%.[1],[2] wAIHA is the most common form of AIHA,[3] accounting for about 75-80% of all adult AIHA cases.[4]
ESLIM-02 is a randomized, double blind, placebo-controlled China Phase II/III study in adult patients with primary or secondary wAIHA who had relapsed or were refractory to at least one prior line of standard treatment. Results from the Phase II part of the study published in The Lancet Haematology in January 2025 demonstrated encouraging hemoglobin benefit compared with placebo, with overall response rate of 43.8% vs 0% in the first 8 weeks, and overall response rate of 66.7% during the 24 weeks of sovleplenib treatment (including patients that crossed over from placebo) with a favorable safety profile.[5] Additional details may be found at clinicaltrials.gov, using identifier NCT05535933.
Professor Fengkui Zhang of the Chinese Academy of Medical Sciences Blood Diseases Hospital, and one of the leading principal investigators of the ESLIM-02 study, said: “Warm antibody autoimmune hemolytic anemia is a highly heterogeneous and often chronically relapsing disease. Patients often experience symptoms like fatigue significantly impacting patients’ quality of life. In severe cases, the disease can become life‑threatening if not managed effectively. The positive topline results from ESLIM-02 highlight sovleplenib’s potential to deliver rapid and durable hemoglobin responses in wAIHA patients who have limited options after failing standard therapies. This could represent a meaningful advancement for managing this challenging condition.”
Professor Bin Han of Peking Union Medical College Hospital and Professor Liansheng Zhang of The Second Hospital of Lanzhou University were also co-leading Principal Investigators of the study. Full results of the ESLIM-02 study will be submitted for presentation at an upcoming scientific conference. HUTCHMED plans to submit the New Drug Application (“NDA”) for sovleplenib for wAIHA to the China National Medical Products Administration (NMPA) in the first half of 2026.
About Sovleplenib and wAIHA
Sovleplenib is a novel, investigational, selective small molecule inhibitor for oral administration targeting the spleen tyrosine kinase, also known as Syk. Syk is a major component in B-cell receptor and Fc receptor signaling and is an established target for the treatment of multiple subtypes of B-cell lymphomas and autoimmune disorders.
The accelerated clearance of antibody-coated RBCs by immunoglobulin Fc-gamma receptor (FcγR) bearing macrophages is thought to be the pathogenic mechanism in wAIHA.[6] Activated Syk mediates downstream signaling of the activated Fc receptors in phagocytic cells, resulting in phagocytosis of RBCs.[7] In addition, activation of Syk through the B-cell receptor mediates activation and differentiation of B-lymphocytes into antibody secreting plasma cells.[8] Inhibition of Syk may have potential effects in the treatment of wAIHA through inhibition of phagocytosis and reduction of antibody production.
In addition to wAIHA, sovleplenib is also being studied in immune thrombocytopenia (“ITP”). Positive results from ESLIM-01 (NCT05029635), a Phase III trial in China of sovleplenib in patients with primary ITP, have been published in The Lancet Haematology. An NDA resubmission for sovleplenib for second-line ITP is planned in the first half of 2026.
HUTCHMED currently retains all rights to sovleplenib worldwide.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the therapeutic potential of sovleplenib for the treatment of wAIHA and the further development of sovleplenib in this and other indications. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding the timing and outcome of clinical studies and the sufficiency of clinical data to support a new drug application submission of sovleplenib for the treatment of wAIHA or other indications in China or other jurisdictions, its potential to gain approvals from regulatory authorities on an expedited basis or at all, the efficacy and safety profile of sovleplenib, HUTCHMED’s ability to fund, implement and complete its further clinical development and commercialization plans for sovleplenib and the timing of these events. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
References
[1] Eaton WW, Rose NR, Kalaydjian A, Pedersen MG, Mortensen PB. Epidemiology of autoimmune diseases in Denmark. J Autoimmun. 2007; 29 (1):1-9. doi: 10.1016/j.jaut.2007.05.002.
[2] Roumier M, Loustau V, Guillaud C, et al. Characteristics and outcome of warm autoimmune hemolytic anemia in adults: new insights based on a single-center experience with 60 patients. Am J Hematol. 2014; 89 (9):E150-5. doi: 10.1002/ajh.23767.
[3] Cotran Ramzi S, Kumar Vinay, Fausto Nelson, Nelso Fausto, Robbins Stanley L, Abbas Abul K. Robbins and Cotran pathologic basis of disease. St. Louis, Mo: Elsevier Saunders; 2005. p. 637.
[4] Gehrs BC, Friedberg RC. Autoimmune haemolytic anemia. Am J Hematol. 2002; 69:258–271. doi: 10.1002/ajh.10062.
[5] Zhao X, Sun J, Zhang Z, et al. Sovleplenib in patients with primary or secondary warm autoimmune haemolytic anaemia: results from phase 2 of a randomised, double-blind, placebo-controlled, phase 2/3 study. Lancet Haematol. 2025;12(2):e97-e108. doi:10.1016/S2352-3026(24)00344-2
[6] Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transfus Med Rev. 2010; 24(3):195‐210. doi: 10.1016/j.tmrv.2010.03.002.
[7] Barcellini W, Fattizzo B, Zaninoni A. Current and emerging treatment options for autoimmune hemolytic anemia. Expert Rev Clin Immunol. 2018; 14(10):857‐872. doi: 10.1080/1744666x.2018.1521722.
[8] Davidzohn N, Biram A, Stoler‐Barak L, Grenov A, Dassa B, Shulman Z. SYK degradation restrains plasma cell formation and promotes zonal transitions in germinal centers. J Exp Med. 2020; 217(3):e20191043. doi: 10.1084/jem.20191043.
Hong Kong, Shanghai & Florham Park, NJ — Monday, January 5, 2026: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that it has initiated the Phase III part of the Phase II/III trial to evaluate the efficacy of the combination of surufatinib, camrelizumab, nab-paclitaxel and gemcitabine as a first-line treatment for patients with metastatic pancreatic ductal adenocarcinoma (“PDAC”) in China. The first patient received the first dose on December 30, 2025.
PDAC is a highly aggressive form of cancer, representing over 90% of pancreatic cancer cases. Globally, an estimated 511,000 people were diagnosed with pancreatic cancer, leading to approximately 467,000 deaths in 2022, with an average five-year survival rate of less than 10%. In China, an estimated 119,000 people were diagnosed with pancreatic cancer, causing approximately 106,000 deaths in 2022.[1] Treatments such as chemotherapy, surgery and radiation are commonly employed, but have not shown significant improvement in patient outcomes. Under 20% of metastatic pancreatic cancer patients survive for more than a year. [2]
The trial is a multicenter, randomized, open-label, active-controlled Phase II/III study to evaluate the efficacy and safety of surufatinib combined with camrelizumab, nab-paclitaxel and gemcitabine (“S+C+AG”) versus nab-paclitaxel plus gemcitabine (“AG”) in adults with metastatic pancreatic cancer who have not previously received systemic anti-tumor therapy. A total of 62 patients were enrolled in the Phase II part, with plans to enroll approximately 400 additional patients in the Phase III part. The primary endpoint for the Phase III part is overall survival (OS). Secondary endpoints include progression-free survival (“PFS”), objective response rate (“ORR”), duration of response (DoR), disease control rate (“DCR”), quality of life and safety. Professor Shukui Qin of China Pharmaceutical University Nanjing Tianyinshan Hospital and Professor Jihui Hao of Tianjin Medical University Cancer Institute and Hospital are the leading principal investigators of this study. Additional details may be found at clinicaltrials.gov, using identifier NCT06361888.
Results from the Phase II part were recently presented at the 2025 European Society for Medical Oncology (ESMO) Asia Congress.[3] As of the data cut-off of July 24, 2025, the median PFS follow-up duration was 8.15 months. The S+C+AG regimen demonstrated a median PFS of 7.20 months compared to 5.52 months for the AG arm (stratified hazard ratio [HR] 0.499, log-rank p=0.0407), representing a 50.1% reduction in the risk of progression or death. Consistent benefits were observed across other key efficacy endpoints, including ORR (67.7% vs 41.9%, p=0.0430) and DCR (93.5% vs 71.0%, p=0.0149). Although overall survival data were immature at the time of analysis, a favorable trend was observed (not reached vs 8.48 months, unstratified HR 0.555), with 9 events in the S+C+AG arm (N=31) and 15 events in the AG arm (N=31). The safety profile was manageable. Treatment-emergent adverse events (TEAEs) of grade 3 or above occurred in 80.6% of patients in the S+C+AG arm compared to 61.3% in the AG arm.
About Surufatinib
Surufatinib is a novel, oral angio-immuno kinase inhibitor that selectively inhibits the tyrosine kinase activity associated with vascular endothelial growth factor receptors (VEGFRs) and fibroblast growth factor receptor (FGFR), which both inhibit angiogenesis, and colony stimulating factor-1 receptor (CSF-1R), which regulates tumor-associated macrophages, promoting the body’s immune response against tumor cells. Surufatinib is marketed in China by HUTCHMED under the brand name SULANDA®. HUTCHMED currently retains all rights to surufatinib worldwide.
About Camrelizumab
Camrelizumab (SHR-1210) is a humanized monoclonal antibody targeting the programmed death-1 (PD-1) receptor. Camrelizumab has been approved in China for multiple indications in areas such as lung cancer, liver cancer, esophageal cancer, nasopharyngeal cancer and cervical cancer. Camrelizumab is marketed in China by Hengrui Pharma under the brand name AiRuiKa®.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the therapeutic potential of surufatinib for the treatment of PDAC and the further development of surufatinib in this and other indications. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding the timing and outcome of clinical studies and the sufficiency of clinical data to support a new drug application submission of surufatinib for the treatment of PDAC or other indications in China or other jurisdictions, its potential to gain approvals from regulatory authorities on an expedited basis or at all, the efficacy and safety profile of surufatinib, HUTCHMED’s ability to fund, implement and complete its further clinical development and commercialization plans for surufatinib and the timing of these events. In addition, as certain studies rely on the use of other drug products such as camrelizumab, nab-paclitaxel and gemcitabine as combination therapeutics with surufatinib, such risks and uncertainties include assumptions regarding the safety, efficacy, supply and continued regulatory approval of these therapeutics. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
References
[1] The Global Cancer Observatory, China fact sheet. https://gco.iarc.who.int/media/globocan/factsheets/populations/160-china-fact-sheet.pdf. Accessed December 3, 2025
[2] Sarantis P et al. Pancreatic ductal adenocarcinoma: Treatment hurdles, tumor microenvironment and immunotherapy. World J Gastrointest Oncol. 2020;12(2):173-181. DOI:10.4251/wjgo.v12.i2.173
[3] Qin S et al. 375P – Surufatinib (S) in combination with camrelizumab (C), nab-paclitaxel and gemcitabine (AG) as the first-line treatment in metastatic pancreatic cancer: Results from phase II part of a randomized, open-label, active-controlled, phase II/III study. Annals of Oncology (2025) 36 (suppl_4): S1859-S1939. 10.1016/annonc/annonc1989
Hong Kong, Shanghai & Florham Park, NJ — Wednesday, December 31, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) hereby notifies the market that as at December 31, 2025, the issued share capital of HUTCHMED consisted of 872,327,620 ordinary shares of US$0.10 each, with each share carrying one right to vote and with no shares held in treasury.
The above figure of 872,327,620 may be used by shareholders as the denominator for the calculations by which they could determine if they are required to notify their interest in, or a change to their interest in, HUTCHMED shares under the Financial Conduct Authority’s Disclosure Guidance and Transparency Rules.
For illustrative purposes only, the 872,327,620 ordinary shares would be equivalent to 872,327,620 depositary interests (each equating to one ordinary share) which are traded on AIM or, if the depositary interests were converted in their entirety, equivalent to 174,465,524 American depositary shares (each equating to five ordinary shares) which are traded on Nasdaq.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
— NDA supported by positive Phase II registration study data in Chinese patients; follows Breakthrough Therapy Designation granted in 2023 —
— Savolitinib has the potential to become the first selective MET inhibitor in China for MET-amplified gastric cancer —
Hong Kong, Shanghai & Florham Park, NJ — Tuesday, December 30, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that the New Drug Application (“NDA”) for savolitinib for the treatment of locally advanced or metastatic gastric cancer or gastroesophageal junction (GC/GEJ) adenocarcinoma patients with MET amplification who have failed at least two prior systemic treatments has been accepted and granted priority review by the China National Medical Products Administration (“NMPA”).
This NDA is supported by data from a single-arm, multi-center, open-label, Phase II registration study of savolitinib in gastric cancer patients with MET amplification in China. The study has met its primary endpoint of objective response rate (ORR) by the Independent Review Committee (IRC) (RECIST 1.1). Additional details may be found at clinicaltrials.gov using identifier NCT04923932.
Gastric cancer remains one of the most common cancers and leading causes of cancer death in China. MET-driven gastric cancer has a very poor prognosis.[1] It is estimated that MET amplification accounts for approximately 4-6% of gastric cancer patients.[2],[3] The annual incidence of MET amplification gastric cancer is estimated to be approximately 18,000 in China.[4]
The NMPA has granted Breakthrough Therapy Designation to savolitinib for this potential indication in 2023. The NMPA granted this designation to this new treatment that could target a serious condition where clinical evidence demonstrates substantial advantages over existing therapies.
About Savolitinib
Savolitinib is an oral, potent, and highly selective MET tyrosine kinase inhibitor (TKI) being jointly developed by AstraZeneca and HUTCHMED and commercialized by AstraZeneca. MET is a tyrosine kinase receptor that has an essential role in normal cell development.[5] Savolitinib blocks atypical activation of the MET receptor tyrosine kinase pathway that occurs because of mutations (such as exon 14 skipping alterations or other point mutations), gene amplification or protein overexpression.
Savolitinib is approved in China and is marketed under the brand name ORPATHYS® by our partner, AstraZeneca, representing the first selective MET inhibitor approved in China. It has been included in the National Reimbursement Drug List of China (NRDL) since March 2023.
It is currently under clinical development for multiple tumor types, including lung, kidney, and gastric cancers as a single treatment and in combination with other medicines.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the review of a NDA for savolitinib for the treatment of gastric cancer with the NMPA and the timing of such review, therapeutic potential of savolitinib for the treatment of patients with gastric cancer and the further development of savolitinib in this and other indications. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding the timing and outcome of clinical studies and the sufficiency of clinical data to support NDA approval of savolitinib for the treatment of patients with gastric cancer or other indications in China or other jurisdictions, its potential to gain approvals from regulatory authorities on an expedited basis or at all, the safety profile of savolitinib, HUTCHMED and/or its partner’s ability to fund, implement and complete its further clinical development and commercialization plans for savolitinib and the timing of these events. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
References
[1] Catenacci DV, Ang A, Liao WL, et al. MET tyrosine kinase receptor expression and amplification as prognostic biomarkers of survival in gastroesophageal adenocarcinoma. Cancer. 2017;123(6):1061-1070. doi:10.1002/cncr.30437
[2] Lee J, Kim ST, Kim K, et al. Tumor Genomic Profiling Guides Patients with Metastatic Gastric Cancer to Targeted Treatment: The VIKTORY Umbrella Trial. Cancer Discov. 2019;9(10):1388-1405. doi:10.1158/2159-8290.CD-19-044
[3] Van Cutsem E, Karaszewska B, Kang YK, et al. A Multicenter Phase II Study of AMG 337 in Patients with MET-Amplified Gastric/Gastroesophageal Junction/Esophageal Adenocarcinoma and Other MET-Amplified Solid Tumors. Clin Cancer Res. 2019;25(8):2414-2423. doi:10.1158/1078-0432.CCR-18-1337
[4] Global Cancer Observatory. China Fact Sheet. https://gco.iarc.who.int/media/globocan/factsheets/populations/160-china-fact-sheet.pdf. Accessed April 7, 2025.
[5] Uchikawa E, et al. Structural basis of the activation of c-MET receptor. Nat Commun. 2021;12(4074).
Hong Kong, Shanghai & Florham Park, NJ — Monday, December 29, 2025: HUTCHMED (China) Limited (“HUTCHMED” or the “Company”) (Nasdaq/AIM:HCM; HKEX:13) announces the following blocklisting six monthly return:
| 1. | Name of applicant: | HUTCHMED (China) Limited | |
| 2. | Name of scheme: | Share Option Scheme conditionally adopted by HUTCHMED in 2015 (“2015 HUTCHMED Share Option Scheme”) | |
| 3. | Period of return: | From June 29, 2025 to December 28, 2025 | |
| 4. | Balance under scheme from previous return: | 2015 HUTCHMED Share Option Scheme: 45,992,018 ordinary shares of US$0.1 each | |
| 5. | The amount by which the block scheme has been increased, if the scheme has been increased since the date of the last return: | 2015 HUTCHMED Share Option Scheme: Nil | |
| 6. | Number of securities issued/allotted under scheme during period: | 2015 HUTCHMED Share Option Scheme: 216,150 | |
| 7. | Balance under scheme not yet issued/allotted at end of the period: | 2015 HUTCHMED Share Option Scheme: 45,775,868 ordinary shares of US$0.1 each | |
| 8. | Number and class of securities originally listed and the date of admission: | 25,198,880 ordinary shares of US$0.1 each admitted on June 17, 2019 (to replace the Company’s previous block admission schemes following the Company’s share subdivision which took effect on May 30, 2019) | |
| 9. | Total number of securities in issue at the end of the period: | 872,327,620 ordinary shares of US$0.1 each | |
| Name of contact: | Weiguo Su / Johnny Cheng | ||
| Address of contact: | Level 18, The Metropolis Tower, 10 Metropolis Drive, Hung Hom, Kowloon, Hong Kong | ||
| Telephone number of contact: | +852 2121 8200 | ||
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch-med.com or follow us on LinkedIn.
— NDA supported by results from a Phase II registration trial in China —
— Second most common form of liver cancer after hepatocellular carcinoma, with generally poorer long-term survival in comparison —
Hong Kong, Shanghai & Florham Park, NJ — Monday, December 29, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that the New Drug Application (“NDA”) for fanregratinib (HMPL-453) for the treatment of adult patients with advanced, metastatic or unresectable intrahepatic cholangiocarcinoma (“ICC”) with fibroblast growth factor receptor (“FGFR”) 2 fusion/rearrangement who have previously received systemic therapy has been accepted and granted priority review by the China National Medical Products Administration (“NMPA”).
Fanregratinib (HMPL‑453) is a novel, selective, oral inhibitor targeting FGFR 1/2/3. ICC is a highly aggressive malignancy arising from the intrahepatic biliary epithelium. It accounts for 8.2-15.0% of primary liver cancers, and consequently it is the second most common type after hepatocellular carcinoma. In recent years, the incidence of ICC has continued to rise, with a 5-year overall survival rate of approximately 9%.[1] Approximately 10-15% of ICC patients globally have tumors harboring FGFR2 fusions or rearrangements.[2],[3]
This NDA is supported by data from a single-arm, multi-center, open-label, Phase II registration study in China. The study has met its primary endpoint of objective response rate (ORR). Results from the secondary endpoints including progression-free survival (PFS), disease control rate (DCR), duration of response (DoR) and overall survival (OS) also support the primary endpoint findings. Full results will be submitted for presentation at an upcoming scientific conference. Additional details may be found at clinicaltrials.gov using identifier NCT04353375.
About Fanregratinib
Fanregratinib (HMPL‑453) is a novel, highly selective and potent inhibitor targeting FGFR 1, 2 and 3. Aberrant FGFR signaling has been found to be a driving force in tumor growth, promotion of angiogenesis and resistance to anti-tumor therapies. Abnormal FGFR gene alterations are believed to be the drivers of tumor cell proliferation in several solid tumor settings. HUTCHMED currently retain all rights to fanregratinib worldwide.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the review of a NDA for fanregratinib for the treatment of ICC with the NMPA and the timing of such review, therapeutic potential of fanregratinib for the treatment of patients with ICC and the further development of fanregratinib in this and other indications. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding the timing and outcome of clinical studies and the sufficiency of clinical data to support NDA approval of fanregratinib for the treatment of patients with ICC or other indications in China or other jurisdictions, its potential to gain approvals from regulatory authorities on an expedited basis or at all, the safety profile of fanregratinib, HUTCHMED’s ability to fund, implement and complete its further clinical development and commercialization plans for fanregratinib and the timing of these events. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
References
[1] Expert consensus on precision detection of intrahepatic cholangiocarcinoma (2024 edition). Chin J Clin Med. 2025;32(1):1-18.
[2] Arai Y, Totoki Y, Hosoda F, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology. 2014;59:1427–34.
[3] Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003–10.
— First-in-human trial of candidate from the next-generation ATTC platform —
— Simultaneous China and global clinical development strategy to expedite development process —
Hong Kong, Shanghai & Florham Park, NJ — Wednesday, December 17, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces the initiation of its global Phase I clinical development program for HMPL-A251, a first-in-class PI3K/PIKK-HER2 Antibody-Targeted Therapy Conjugate (“ATTC”) comprising a highly selective and potent PI3K/PIKK inhibitor payload conjugated to a humanized anti-HER2 IgG1 antibody via a cleavable linker. Study sites are in the US and China. The first patient received the first dose on December 16, 2025, in China.
This first-in-human Phase I/IIa, open-label, multicenter clinical study evaluates HMPL-A251 monotherapy in adult patients with unresectable, advanced or metastatic HER2-expressing solid tumors. The study is divided into two parts, a Phase I dose escalation part and a Phase IIa dose expansion and optimization part. The primary outcome measures are to evaluate the safety and tolerability of HMPL-A251 and to determine the maximum tolerated dose (MTD) and/or recommended dose(s) for expansion (“RDE”) in the Phase I part, and to further evaluate safety and preliminary efficacy at RDEs and to determine the recommended dose for Phase II (RP2D) or Phase III (RP3D) in the Phase IIa part. Secondary outcome measures include preliminary antitumor activity, pharmacokinetic profile, and the immunogenicity of HMPL-A251. Additional details may be found at clinicaltrials.gov, using identifier NCT07228247.
HMPL-A251 is the first clinical-stage candidate derived from HUTCHMED’s next-generation ATTC platform. The first family of programs are based on a highly potent and selective PI3K/PIKK inhibitor payload. By conjugating this highly novel payload to an anti-HER2 antibody, the molecule is designed to deliver targeted pathway inhibition directly into HER2-expressing tumor cells, thereby potentially overcoming the systemic toxicity and narrow therapeutic index historically associated with PI3K/PIKK inhibitors. This approach aims to achieve deeper and more durable target inhibition while improving the overall tolerability profile.
Preclinical data for HMPL-A251 were presented at the 2025 AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics. This body of evidence supports the translational potential of the ATTC platform, the ongoing global clinical evaluation of HMPL-A251, and the broad potential of HUTCHMED’s PI3K/PIKK inhibitor linker-payload to underpin a family of future ATTC drug candidates.
About the ATTC Platform
HUTCHMED’s ATTC platform represents a next-generation approach to precision oncology, combining monoclonal antibodies with proprietary small-molecule inhibitor payloads to deliver dual mechanisms of action. Unlike traditional cytotoxin-based Antibody Drug Conjugates, ATTCs combine targeted therapies to achieve synergistic anti-tumor activity and durable responses in preclinical models, outperforming standalone antibody or small-molecule inhibitor components in efficacy and safety.
Built on over 20 years of targeted therapy expertise, the platform enables development of drug candidates for diverse cancer types. By leveraging antibody-guided delivery and tumor-specific payload release, ATTCs improve the accessibility to tumors and reduce off-tumor toxicity. This overcomes challenges of traditional small-molecule inhibitors, ensures safer long-term use, and supports combinations with chemotherapy and immunotherapy, unlocking potential for early-line treatments.
About the PAM Pathway and HMPL-A251
The PI3K/AKT/mTOR (“PAM”) pathway is a critical intracellular network involved in cell growth, survival, and division. Alterations in the PAM pathway are frequently associated with poor prognosis and resistance to treatment across various cancers. However, existing PAM-targeted drugs face significant challenges, including on-target toxicities that restrict dosing, feedback loops that enable pathway reactivation, and insufficient tumor-specific delivery. HUTCHMED has designed a highly novel PI3K/PIKK inhibitor linker-payload to overcome these challenges with broad potential to lead to a family of antibody conjugate drug candidates.
HMPL-A251 is a first-in-class ATTC comprising of this highly selective and potent PI3K/PIKK inhibitor payload conjugated to a humanized anti-HER2 IgG1 antibody via a cleavable linker, designed to address challenges by enhancing targeted delivery directly to tumor cells, maximizing therapeutic benefit while minimizing systemic exposure. In preclinical studies, the HMPL-A251 payload exhibited high selectivity, potency, and robust anti-tumor activity. HMPL-A251 exhibited superior anti-tumor efficacy and tolerability compared to co-administration of the naked antibody and payload.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the therapeutic potential of HMPL‑A251 and other drug candidates from the ATTC platform and the further development of HMPL‑A251 and other drug candidates from the ATTC platform in this and other indications. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding the timing and outcome of clinical studies and the sufficiency of clinical data to support an new drug application submission of HMPL‑A251 and other drug candidates from the ATTC platform in China or other jurisdictions, its potential to gain approvals from regulatory authorities on an expedited basis or at all, the efficacy and safety profile of HMPL‑A251 and other drug candidates from the ATTC platform, HUTCHMED’s ability to fund, implement and complete its further clinical development and commercialization plans for HMPL‑A251 and other drug candidates from the ATTC platform and the timing of these events. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Hong Kong, Shanghai & Florham Park, NJ — Monday, December 8, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that following the contract renewal with the China National Healthcare Security Administration (“NHSA”), the updated National Reimbursement Drug List (“NRDL”) effective on January 1, 2026 will continue to include ELUNATE®, ORPATHYS® and SULANDA®. In addition, TAZVERIK® will be included in the first edition of the National Commercial Health Insurance Innovative Drug List (“Commercial Insurance Drug List”).
ELUNATE® (fruquintinib) is included for the treatment of patients with advanced endometrial cancer with Mismatch Repair proficient (pMMR) tumors that have failed prior systemic therapy and are not candidates for curative surgery or radiation, in combination with TYVYT® (sintilimab injection). It is also renewed for the treatment of patients with metastatic colorectal cancer who have previously received fluoropyrimidine, oxaliplatin and irinotecan-based chemotherapy, and those who have previously received or are not suitable for receiving anti-VEGF therapy or anti-epidermal growth factor receptor (EGFR) therapy (RAS wild-type).
ORPATHYS® (savolitinib) is included for treatment of adult patients with locally advanced or metastatic non-small cell lung cancer with MET exon 14 skipping alteration.
SULANDA® (surufatinib) is renewed for the treatment of patients with unresectable; locally advanced or metastatic; progressive non-functional, well-differentiated (G1 or G2) pancreatic and non-pancreatic neuroendocrine tumors.
TAZVERIK® (tazemetostat) is included in the Commercial Insurance Drug List for the treatment of adult patients with relapsed or refractory follicular lymphoma with EZH2 mutation who have received at least two prior systemic therapies. In July 2025, the NHSA issued the 2025 Adjustment Work Plan for the NRDL and the Commercial Health Insurance Innovative Drug List, announcing the establishment of the new Commercial Insurance Drug List. This list, together with the NRDL, forms a key component of China’s multi-level medical insurance system. This new list focuses on medicines with high innovation and significant clinical value that fall beyond the scope of basic medical insurance, including certain high-cost oncology drugs, gene therapies, and rare disease therapies, enabling reimbursement through commercial health insurance products such as high-limit medical insurance, inclusive health plans (“Huiminbao”) and group health insurance. This multi-layered reimbursement framework enhances patient access to breakthrough treatments while supporting the sustainable development of China’s innovative pharmaceutical sector.
About NRDL
The government in China has placed great importance on improving the affordability of drug treatments for the public. As of end of 2024, 1.33 billion people in China had basic medical insurance coverage, representing around 95% of the entire population. The NRDL is updated every year, and inclusion on the list is subject to renewal every two years. The NHSA annually convenes a broad network of experts in medicine, pharmacology, pharmacoeconomics and actuarial valuation to identify innovative medicines to consider for NRDL inclusion. Reimbursement of Category B medicines, including novel oncology medicines, requires varying degrees of copayment from patients, depending on their provinces or types of NHSA insurance scheme enrollment.
About Fruquintinib
Fruquintinib is a selective oral inhibitor of all three vascular endothelial growth factor receptors (“VEGFR”) -1, ‑2 and -3. Fruquintinib is co-developed and co-commercialized in China by HUTCHMED and Eli Lilly and Company under the brand name ELUNATE®. Takeda holds the exclusive worldwide license to further develop, commercialize, and manufacture fruquintinib outside mainland China, Hong Kong and Macau, marketing it under the brand name FRUZAQLA®.
About Savolitinib
Savolitinib is an oral, potent and highly selective MET tyrosine kinase inhibitor that has demonstrated clinical activity in advanced solid tumors. It blocks atypical activation of the MET receptor tyrosine kinase pathway that occurs because of mutations (such as exon 14 skipping alterations or other point mutations), gene amplification or protein overexpression. Savolitinib is being jointly developed by AstraZeneca and HUTCHMED, and commercialized by AstraZeneca under the brand name ORPATHYS®.
About Surufatinib
Surufatinib is a novel, oral angio-immuno kinase inhibitor that selectively inhibits the tyrosine kinase activity associated with VEGFRs and fibroblast growth factor receptor (FGFR), which both inhibit angiogenesis, and colony stimulating factor-1 receptor (CSF-1R), which regulates tumor-associated macrophages, promoting the body’s immune response against tumor cells. Surufatinib is marketed in China by HUTCHMED under the brand name SULANDA®. HUTCHMED currently retains all rights to surufatinib worldwide.
About Tazemetostat
Tazemetostat is a first-in-class methyltransferase inhibitor of EZH2 developed by Epizyme, an Ipsen company. HUTCHMED entered into a strategic collaboration with Epizyme to research, develop, manufacture and commercialize tazemetostat in Chinese Mainland, Hong Kong, Macau and Taiwan.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations for the commercialization of fruquintinib, savolitinib, surufatinib and tazemetostat in China, the potential benefits and further clinical development of fruquintinib, savolitinib, surufatinib and tazemetostat, its expectations as to whether further studies would meet their primary or secondary endpoints, and its expectations as to the timing of the completion and the release of results from such studies. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding the commercial acceptance of fruquintinib, savolitinib, surufatinib and tazemetostat, the impact of the inclusion of fruquintinib, savolitinib and surufatinib on the NRDL and tazemetostat on the Commercial Health Insurance Innovative Drug List on sales of the drug and its pricing, clinical trial enrollment rates, timing and availability of subjects meeting a study’s inclusion and exclusion criteria, changes to clinical protocols or regulatory requirements, unexpected adverse events or safety issues, the ability of fruquintinib, savolitinib, surufatinib and tazemetostat to obtain regulatory approval for a targeted indication in different jurisdictions and the sufficiency of funding. In addition, as certain studies rely on the use of other drug products such as sintilimab as combination therapeutics, such risks and uncertainties include assumptions regarding their safety, efficacy, supply and continued regulatory approval. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
Hong Kong, Shanghai & Florham Park, NJ — Thursday, November 27, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that new and updated data from several studies of compounds discovered by HUTCHMED will be presented at the European Society for Medical Oncology (“ESMO”) Asia Congress 2025, taking place on December 5-7, 2025 in Singapore, and the American Society of Hematology (“ASH”) Annual Meeting taking place on December 6-9, 2025 in Orlando, USA.
Results from a first-in-human study of the anti-CD47 monoclonal antibody HMPL-A83 in advanced solid tumors, as well as from the phase II part of the FRUSICA-2 registration study of the fruquintinib and sintilimab combination as a second-line treatment for locally advanced or metastatic renal cell carcinoma, will be presented at the ESMO Asia Congress 2025. Results from the phase II part of the phase II/III study of surufatinib in combination with camrelizumab and chemotherapy as a first-line treatment for metastatic pancreatic cancer will also be reported. Details of the presentations are as follows:
| Abstract title | Presenter/Lead author | Presentation details |
| ESMO Asia Congress 2025 – SPONSORED STUDIES | ||
| A first-in-human (FIH), dose escalation study of HMPL-A83 (A83), an anti-CD47 monoclonal antibody (mAb) in patients (pts) with advanced solid tumors |
Ye Guo (Shanghai, China) |
162MO | Mini Oral session: Developmental therapeutics and precision medicine Sunday, December 7, 2025 11:40 – 11:45 SGT Hall 407 |
| Fruquintinib monotherapy as second-line (2L) treatment in locally advanced or metastatic renal cell carcinoma (RCC): results from phase 2 part of FRUSICA-2 | Shanshan Wang (Shanghai, China) |
540O | Proffered Paper session: Genitourinary tumours Friday, December 5, 2025 14:55 – 15:05 SGT Hall 402 |
| Surufatinib (S) in combination with camrelizumab (C), nab-paclitaxel and gemcitabine (AG) as the first-line treatment in metastatic pancreatic cancer: results from phase 2 part of a randomized, open-label, active-controlled, phase 2/3 study | Shukui Qin (Nanjing, China) |
375P | Poster Display: Gastrointestinal tumours, non‑colorectal |
| Osimertinib (osi) + savolitinib (savo) in EGFR-mutated (EGFRm) advanced non-small cell lung cancer (NSCLC) with MET overexpression and/or amplification (OverExp/Amp) following progressive disease (PD) on osi: SAVANNAH Asian subset | Se-Hoon Lee (Seoul, Korea) |
982P | Poster Display: Thoracic tumours, metastatic |
| Patient-relevant Outcomes (PROs) from SACHI: a Phase 3 Trial of Savolitinib (Savo) plus Osimertinib (Osi) versus Chemotherapy (Chemo) in EGFR-mutant (EGFRm) and MET-amplified (METamp) Advanced NSCLC after Progression on EGFR-TKIs | Yongfeng Yu (Shanghai, China) |
984P | Poster Display: Thoracic tumours, metastatic |
| Analysis of MET Amplification (METamp) with FISH and NGS Method in SACHI Trial | Longhua Sun (Nanchang, China) |
988P | Poster Display: Thoracic tumours, metastatic |
| Progression pattern in patients (pts) with EGFR-mutant (EGFRm), MET-amplified (METamp) advanced NSCLC treated with savolitinib (savo) plus osimertinib (osi) | Haiyan Yang (Changsha, China) |
1002P | Poster Display: Thoracic tumours, metastatic |
| MET testing and treatment (tx) sequencing after progression of disease (PD) on first-line (1L) osimertinib (osi) in patients (pts) with EGFRm advanced NSCLC and acquired MET overexpression and/or amplification (OverExp/Amp): Final analysis of a global real-world (rw) study | Julia Rotow (Boston, US) |
1005P | Poster Display: Thoracic tumours, metastatic |
| ESMO Asia Congress 2025 – INVESTIGATOR-INITIATED STUDIES | ||
| Fruquintinib Combined with TAS-102 with or without SBRT as Third- or Later-Line Treatment in Metastatic Colorectal Cancer: Preliminary Results from a Prospective Phase II Trial | Chen Zhang/ Yi Wang (Ningbo, China) |
205P | Poster Display: Gastrointestinal tumours, colorectal |
| Efficacy and safety of fruquintinib combined with PD-1 inhibitor and chidamide in MSS mCRC: a comparison with real-world bevacizumab plus anti-pd-1 and chidamide arm | Guanghai Dai/ Miaomiao Gou (Beijing, China) |
245eP | Poster Display: Gastrointestinal tumours, colorectal |
| The Efficacy and Safety of Fruquintinib (F) Plus FOLFIRI as Second-line (2L) Treatment in Bevacizumab (Bev)-pretreated RAS-mutated (RAS‑m) Metastatic Colorectal Cancer (mCRC) | Zhenyang Liu/ Xiaolin Yang (Changsha, China) |
250eP | Poster Display: Gastrointestinal tumours, colorectal |
| Real-world Observational Study of Fruquintinib in Combination with Irinotecan and Capecitabine as Second-line Treatment in Patients with Advanced Colorectal Cancer | Xiujuan Qu/ Lin Xu (Shenyang, China) |
255eP | Poster Display: Gastrointestinal tumours, colorectal |
| Matching-Adjusted Indirect Comparison of Surufatinib versus High-Dose Octreotide LAR in Advanced Extrapancreatic Neuroendocrine Tumors | Jianming Xu (Beijing, China) |
214P | Poster Display: Gastrointestinal tumours, colorectal |
| Efficacy and safety of surufatinib in combination with CAPTEM as conversion therapy in patients with unresectable pancreatic neuroendocrine tumors (pNETs): Data updates from a prospective, open-label study | Xubao Liu/ Ziyao Wang (Chengdu, China) |
400P | Poster Display: Gastrointestinal tumours, non‑colorectal |
Final analysis of long-term results of sovleplenib’s ESLIM-01 China Phase III study in in adult patients with chronic primary immune thrombocytopenia will be presented at the 2025 ASH Annual Meeting. Details of the presentation is as follows:
| Abstract title | Presenter/Lead author | Presentation details |
| 2025 ASH Annual Meeting – SPONSORED STUDIES | ||
| Phase 3 ESLIM-01 study: Final analysis of efficacy and safety of long-term treatment with sovleplenib in adults with chronic primary immune thrombocytopenia |
Renchi Yang (Tianjin, China) | 843 | Oral Abstract Session Monday, December 8, 2025 15:15 – 15:30 EST Room OCCC – W304EFGH |
About Fruquintinib
Fruquintinib is a selective oral inhibitor of all three vascular endothelial growth factor receptors (“VEGFR”) -1, ‑2 and -3. Fruquintinib is co-developed and co-commercialized in China by HUTCHMED and Eli Lilly and Company under the brand name ELUNATE®. Takeda holds the exclusive worldwide license to further develop, commercialize, and manufacture fruquintinib outside mainland China, Hong Kong and Macau, marketing it under the brand name FRUZAQLA®.
About HMPL-A83
HMPL-A83 is an investigational IgG4-type humanized anti-CD47 monoclonal antibody that exhibits high affinity for CD47. HMPL-A83 blocks CD47 binding to Signal regulatory protein (SIRP) α and disrupts the “do not eat me” signal that cancer cells use to shield themselves from the immune system. HUTCHMED currently retains all rights to HMPL-A83 worldwide.
About Savolitinib
Savolitinib is an oral, potent and highly selective MET tyrosine kinase inhibitor that has demonstrated clinical activity in advanced solid tumors. It blocks atypical activation of the MET receptor tyrosine kinase pathway that occurs because of mutations (such as exon 14 skipping alterations or other point mutations), gene amplification or protein overexpression. Savolitinib is being jointly developed by AstraZeneca and HUTCHMED, and commercialized by AstraZeneca under the brand name ORPATHYS®.
About Surufatinib
Surufatinib is a novel, oral angio-immuno kinase inhibitor that selectively inhibits the tyrosine kinase activity associated with VEGFRs and fibroblast growth factor receptor (FGFR), which both inhibit angiogenesis, and colony stimulating factor-1 receptor (CSF-1R), which regulates tumor-associated macrophages, promoting the body’s immune response against tumor cells. Surufatinib is marketed in China by HUTCHMED under the brand name SULANDA®. HUTCHMED currently retains all rights to surufatinib worldwide.
About Sovleplenib
Sovleplenib is a novel, investigational, selective small molecule inhibitor for oral administration targeting the spleen tyrosine kinase, also known as Syk. Syk is a major component in B-cell receptor and Fc receptor signaling and is an established target for the treatment of multiple subtypes of B-cell lymphomas and autoimmune disorders. HUTCHMED currently retains all rights to sovleplenib worldwide.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including but not limited to its expectations regarding the therapeutic potential of fruquintinib, HMPL-A83, surufatinib, savolitinib and sovleplenib, the further clinical development for fruquintinib, HMPL-A83, surufatinib, savolitinib and sovleplenib, its expectations as to whether any studies on fruquintinib, HMPL-A83, surufatinib, savolitinib and sovleplenib would meet their primary or secondary endpoints, and its expectations as to the timing of the completion and the release of results from such studies. Such risks and uncertainties include, among other things, assumptions regarding enrollment rates and the timing and availability of subjects meeting a study’s inclusion and exclusion criteria; changes to clinical protocols or regulatory requirements; unexpected adverse events or safety issues; the ability of fruquintinib, HMPL-A83, surufatinib, savolitinib and sovleplenib, including as combination therapies, to meet the primary or secondary endpoint of a study, to obtain regulatory approval in different jurisdictions and to gain commercial acceptance after obtaining regulatory approval; the potential markets of fruquintinib, HMPL-A83, surufatinib, savolitinib and sovleplenib for a targeted indication, and the sufficiency of funding. In addition, as certain studies rely on the use of other drug products such as camrelizumab and osimertinib as combination therapeutics, such risks and uncertainties include assumptions regarding their safety, efficacy, supply and continued regulatory approval. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
| Date: Monday, November 17, 2025 |
| Time: 3:30pm GMT (10:30 am EDT/ 11:30 pm HKT) |
Hong Kong, Shanghai & Florham Park, NJ — Wednesday, November 5, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces the completion of patient enrollment of SAFFRON, a global Phase III study of ORPATHYS® (savolitinib) and TAGRISSO® (osimertinib) for the treatment of patients with epidermal growth factor receptor (“EGFR”)-mutated, MET-overexpressed and/or amplified, locally advanced or metastatic non-small cell lung cancer (“NSCLC”) following progression on treatment with TAGRISSO®. The last patient was randomized on October 31, 2025.
This combination represents a promising, chemotherapy-free, all-oral treatment option following progression on an EGFR tyrosine kinase inhibitor (“TKI”), and was granted approval in China in June 2025 based on the results of the SACHI randomized Phase III trial. ORPATHYS® is an oral, potent and highly selective MET TKI being jointly developed by AstraZeneca and HUTCHMED and commercialized by AstraZeneca. TAGRISSO® is a third-generation, irreversible EGFR TKI.
SAFFRON is a global Phase III, open-label, randomized, multicenter study to investigate the efficacy and safety of ORPATHYS® administered orally in combination with TAGRISSO® versus platinum-based doublet chemotherapy in participants with EGFR-mutated, MET-overexpressed and/or amplified, locally advanced or metastatic NSCLC who have progressed on first- or second-line treatment with TAGRISSO® as the most recent therapy. The primary endpoint of the study is progression free survival (PFS) as assessed by blinded independent central review (BICR) according to RECIST 1.1 criteria. Other endpoints include overall survival (OS), objective response rate (“ORR”), duration of response (DoR), disease control rate (DCR), time to response (TTR), and safety. This study randomized 338 patients, screened from over 230 sites across 29 countries. Additional details may be found at clinicaltrials.gov, using identifier NCT05261399.
Topline results from the SAFFRON study are estimated to be reported in the first half of 2026, followed by submission of results for presentation at an appropriate medical congress. If favorable, the results could support global regulatory filings for the ORPATHYS® and TAGRISSO® combination.
About NSCLC and MET aberrations
Lung cancer is the leading cause of cancer death, accounting for about one-fifth of all cancer deaths.[1] Lung cancer is broadly split into NSCLC and small cell lung cancer, with 80-85% classified as NSCLC.[2] The majority of NSCLC patients (approximately 75%) are diagnosed with advanced disease, and approximately 10-15% of NSCLC patients in the US and Europe and up to 40-50% of patients in Asia have EGFR-mutated (“EGFRm”) NSCLC.[3],[4],[5],[6],[7]
MET is a tyrosine kinase receptor that has an essential role in normal cell development. MET overexpression and/or amplification can lead to tumor growth and the metastatic progression of cancer cells, and is one of the mechanisms of de novo or acquired resistance to EGFR TKI for metastatic EGFRm NSCLC.[8],[9]
About ORPATHYS®
ORPATHYS® (savolitinib) is an oral, potent and highly selective MET TKI that has demonstrated clinical activity in advanced solid tumors. It blocks atypical activation of the MET receptor tyrosine kinase pathway that occurs because of mutations (such as exon 14 skipping alterations or other point mutations), gene amplification or protein overexpression.
ORPATHYS® is approved in China and is marketed by AstraZeneca for the treatment of adult patients with locally advanced or metastatic NSCLC with MET exon 14 skipping alterations, representing the first selective MET inhibitor approved in China. ORPATHYS® is also approved in China for the treatment of patients with locally advanced or metastatic EGFRm-positive non-squamous NSCLC with MET amplification after disease progression on EGFR tyrosine kinase inhibitor therapy, in combination with TAGRISSO®.
It is currently under clinical development for multiple tumor types, including lung, kidney, and gastric cancers as a single treatment and in combination with other medicines.
About TAGRISSO®
TAGRISSO® (osimertinib) is a third-generation, irreversible EGFR-TKI with proven clinical activity in NSCLC, including against central nervous system (CNS) metastases. TAGRISSO® (40mg and 80mg once-daily oral tablets) has been used to treat more than one million patients across its indications worldwide and AstraZeneca continues to explore TAGRISSO® as a treatment for patients across multiple stages of EGFRm NSCLC.
There is an extensive body of evidence supporting the use of TAGRISSO® in EGFRm NSCLC, and it is the only targeted therapy shown to improve patient outcomes across all stages of the disease.
In late-stage disease, TAGRISSO® demonstrated improved outcomes as monotherapy in the FLAURA Phase III trial and in combination with chemotherapy in the FLAURA2 Phase III trial. TAGRISSO® is also being investigated in this setting in combination with ORPATHYS® (savolitinib) in the SAFFRON Phase III trial and in combination with DATROWAY® (datopotamab deruxtecan or Dato-DXd) in the TROPION-Lung14 and TROPION-Lung15 Phase III trials.
TAGRISSO® also showed improved outcomes in early-stage disease in the NeoADAURA and ADAURA Phase III trials and in locally advanced stages in the LAURA Phase III trial. As part of AstraZeneca’s ongoing commitment to treating patients as early as possible in lung cancer, TAGRISSO® is also being investigated in the early-stage adjuvant resectable setting in the ADAURA2 Phase III trial.
About ORPATHYS® and TAGRISSO® Combination Development in EGFR-mutated NSCLC
This combination represents a promising chemotherapy-free oral treatment strategy to address mechanisms of resistance in this setting. Among patients who experience disease progression following treatment with a third-generation EGFR TKI, approximately 15-50% present with MET aberration, depending on the sample type, detection method and assay cut-off used. TAGRISSO® is a third-generation, irreversible EGFR-TKI with proven clinical activity in NSCLC, including against central nervous system metastases. Treatment with ORPATHYS® in combination with TAGRISSO® has been studied extensively in these patients in the TATTON study (NCT02143466) and the SAVANNAH single-arm Phase II study (NCT03778229). Strong data from SAVANNAH presented at the 2025 European Lung Cancer Congress (ELCC) demonstrated high, clinically meaningful and durable ORR, with consistent safety results. The encouraging results led to the initiation of several randomized Phase III trials in this setting including the SACHI trial in China (NCT05015608) and the global SAFFRON trial (NCT05261399), as well as the SANOVO trial in China (NCT05009836).
SACHI: This Phase III trial in China evaluated the combination of ORPATHYS® and TAGRISSO® compared to platinum-based doublet chemotherapy for the treatment of patients with EGFRm, MET-amplified locally advanced or metastatic NSCLC following progression on treatment with an EGFR TKI. Results were presented at the 2025 ASCO Annual Meeting. The treatment combination received approval in China in June 2025.
SAFFRON: This ongoing global Phase III trial is to evaluate the combination of ORPATHYS® and TAGRISSO® compared to platinum-based doublet chemotherapy in patients with EGFRm, MET-overexpressed and/or amplified, locally advanced or metastatic NSCLC following progression on treatment with TAGRISSO®. This received Fast Track Designation from the US Food and Drug Administration (FDA) and enrollment was completed in October 2025. We look forward to completing this trial to support potential US and other global registration filings.
SANOVO: This ongoing Phase III trial in China is to evaluate the combination of ORPATHYS® and TAGRISSO® compared to TAGRISSO® monotherapy in previously untreated patients with locally advanced or metastatic NSCLC with EGFRm and MET overexpression. Enrollment was completed in August 2025.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the therapeutic potential of ORPATHYS®, the further clinical development for ORPATHYS®, its expectations as to whether any studies on ORPATHYS® would meet their primary or secondary endpoints, and its expectations as to the timing of the completion and the release of results from such studies. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding enrollment rates and the timing and availability of subjects meeting a study’s inclusion and exclusion criteria; changes to clinical protocols or regulatory requirements; unexpected adverse events or safety issues; the ability of ORPATHYS®, including as a combination therapy, to meet the primary or secondary endpoint of a study, to obtain regulatory approval in other jurisdictions and to gain commercial acceptance after obtaining regulatory approval; the potential market of ORPATHYS® for a targeted indication; and HUTCHMED and/or its partner’s ability to fund, implement and complete its further clinical development and commercialization plans for ORPATHYS®, and the timing of these events. In addition, as certain studies rely on the use of other drug products such as TAGRISSO® as combination therapeutics with ORPATHYS®, such risks and uncertainties include assumptions regarding the safety, efficacy, supply and continued regulatory approval of these therapeutics. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
References
[1] World Health Organization. International Agency for Research on Cancer. All cancers fact sheet. Available at: https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf. Accessed November 2022.
[2] American Cancer Society. What is Lung Cancer? Available at: https://www.cancer.org/cancer/lung-cancer/about/what-is.html. Accessed November 2022.
[3] Knight SB, et al. Progress and prospects of early detection in lung cancer. Open Biol. 2017;7(9): 170070.
[4] Keedy VL, et al. American Society of Clinical Oncology Provisional Clinical Opinion: Epidermal Growth Factor Receptor (EGFR) Mutation Testing for Patients with Advanced Non-Small-Cell Lung Cancer Considering First-Line EGFR Tyrosine Kinase Inhibitor Therapy. J Clin Oncol. 2011:29;2121-27.
[5] Zhang Y, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7(48).
[6] Szumera-Ciećkiewicz A, et al. EGFR Mutation Testing on Cytological and Histological Samples in 11. Non-Small Cell Lung Cancer: a Polish, Single Institution Study and Systematic Review of European Incidence. Int J Clin Exp Pathol. 2013:6;2800-12.
[7] Gou LY, et al. Prevalence of driver mutations in non-small-cell lung cancers in the People’s Republic of China. Lung Cancer: Targets and Therapy. 2014; 5; 1–9.
[8] Uchikawa E, et al. Structural basis of the activation of c-MET receptor. Nat Commun. 2021;12(4074).
[9] Wang Q, et al. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. Journal of Hematology & Oncology. 2019;63.
— HUTCHMED unveils its innovative ATTC platform, potentially providing precision oncology with synergistic dual-mechanism of action —
— Lead candidate HMPL-A251 harnesses a selective PI3K/PIKK inhibitor payload, demonstrating promising preclinical efficacy and safety —
— Progress in global and China trials, including FRUSICA-2, SANOVO, surufatinib’s PDAC and fanregratinib’s IHCC studies, advances HUTCHMED’s late-stage pipeline —
Hong Kong, Shanghai & Florham Park, NJ — Monday, November 3, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) announced key research and development (R&D) and business updates presented during its R&D Updates event held on October 31, 2025. The event highlighted HUTCHMED’s progress in advancing innovative cancer and immunology treatments, including the introduction of its next-generation Antibody-Targeted Therapy Conjugate (“ATTC”) platform, alongside updates on late-stage pipeline candidates.
“Our commitment to advancing innovative therapies drives HUTCHMED’s mission to address critical unmet needs in oncology and immunology,” said Dr. Michael Shi, Head of R&D and Chief Medical Officer of HUTCHMED. “The ATTC platform’s potential to redefine precision oncology, combined with our robust pipeline and partnership strategy, positions us to deliver potentially transformative cancer and immunology treatments to patients around the world.”
Breakthrough ATTC Platform and Lead Candidate HMPL-A251
The ATTC platform represents potentially a groundbreaking approach to precision oncology, integrating monoclonal antibodies with proprietary small-molecule inhibitor payloads to deliver dual mechanisms of action. The ATTC platform integrates monoclonal antibodies with proprietary small-molecule inhibitor payloads to deliver dual mechanisms of action. In contrast to traditional cytotoxin-based antibody-drug conjugates (“ADC”), ATTCs leverage targeted therapies to achieve synergistic anti-tumor activity and durable responses, as demonstrated in preclinical models. These conjugates have shown superior efficacy and safety profiles compared to standalone antibody or small molecule inhibitor components.
Overcoming Cancer Challenges with PAM-Targeting Payload: The first wave of ATTC candidates focuses on payloads targeting the PI3K/AKT/mTOR (“PAM”) signaling pathway. The PAM pathway is a critical intracellular network involved in cell growth, survival, and division. Alterations in the PAM pathway are frequently associated with poor prognosis and resistance to treatment across various cancers. However, existing PAM-targeted drugs face significant limitations, including on-target toxicities that restrict dosing, feedback loops that enable pathway reactivation, and insufficient tumor-specific delivery. The ATTC strategy tries to address these challenges by enhancing targeted delivery of PAM inhibitors directly to tumor cells, maximizing therapeutic benefit while minimizing systemic exposure.
HUTCHMED’s Lead ATTC Candidate, HMPL-A251: HMPL-A251 is a PAM-HER2 ATTC consisting of a highly selective and potent PI3K/PIKK inhibitor payload conjugated to a humanized anti-HER2 IgG1 antibody via a cleavable linker. Preclinical data for HMPL-A251 was recently presented at the 2025 AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics. In vitro, the PI3K/PIKK inhibitor payload exhibited high selectivity, potency, and robust anti-tumor activity across a diverse panel of tumor cell lines. HMPL-A251 demonstrated HER2-dependent antitumor activity, with potent inhibition of HER2-positive tumor cell growth regardless of PAM pathway alterations, and moderately reduced activity in HER2-low, PAM-altered lines. Notably, HMPL-A251 exhibited a strong bystander effect, impacting HER2-null cells when co-cultured with HER2-positive cells. In vivo evaluations showed superior anti-tumor efficacy and tolerability compared to the naked antibody and payload administered separately. When benchmarked against trastuzumab deruxtecan (T‑DXd), a leading HER2-directed ADC, HMPL-A251 achieved comparable or superior efficacy at equivalent doses in most models tested. Furthermore, payload-related toxicities are anticipated to be improved, with plasma exposure of the free payload being significantly lower than that of HMPL-A251.
Encouraged by these promising preclinical results in both HER2-positive and HER2-low models, with or without PAM alterations, HUTCHMED plans to advance HMPL-A251 into clinical development starting in late 2025 using a data-driven strategy. Initial studies will evaluate the candidate across various cancer types with diverse HER2 and PAM alteration statuses.
Adaptable Payload and Antibody Design Unlocks Versatile Mechanisms: Beyond HER2-targeted antibodies, HUTCHMED aims to explore a broader range of antibody selections that synergize with the payload’s signaling pathways, leveraging the antibody as a delivery vehicle to enhance combination effects. Payload options are also adaptive, targeting a wide array of signaling pathways, positioning ATTCs as a versatile tool in overcoming resistance and improving treatment outcomes. Additionally, ATTC shows potential for combination with chemotherapy-based frontline standard-of-care treatments or as a chemotherapy-free adjuvant for long-term use, enhancing its possible application as combination therapy in early-line settings. Successful development of multiple ATTC molecules is expected to lead to collaboration and licensing opportunities in the future. Initial responses from potential partners are very positive.
Significant Progress from Late-stage Programs
In addition to the ATTC platform, the R&D Updates featured updates on some of the late-stage programs:
- Fruquintinib FRUSICA-2 Study: Data from the Phase III trial of fruquintinib in combination with sintilimab for second-line renal cell carcinoma was presented at ESMO Congress 2025. The combination achieved a progression-free survival (PFS) of 22.2 months versus 6.9 months with standard-of-care axitinib or everolimus (hazard ratio [HR]: 0.37; p<0.0001). The objective response rate more than doubled to 60.5% versus 24.3%, with a median duration of response of 23.7 months compared to 11.3 months.
- Savolitinib Registration Studies: Recruitment has been completed for the SANOVO China Phase III study in first-line EGFR-mutated non-small cell lung cancer (“NSCLC”) with MET overexpression. Recruitment for the SAFFRON global Phase III study for second-line EGFR-mutated NSCLC patients with MET amplification or overexpression is progressing well, with enrollment completion expected in late 2025.
- Surufatinib for Pancreatic Cancer: The Phase II/III study of surufatinib combined with Hengrui’s camrelizumab (a PD-1 antibody), nab-paclitaxel, and gemcitabine for first-line treatment of metastatic pancreatic ductal adenocarcinoma (PDAC) remains on track. Results from the Phase II portion will be presented at an upcoming scientific conference.
- Sovleplenib for ITP and wAIHA: Preparations for the resubmission of the new drug application for second-line immune thrombocytopenia (ITP) are progressing as outlined in the 2025 interim report, with resubmission planned for the second quarter of 2026. The ESLIM-02 study in second-line warm autoimmune hemolytic anemia (wAIHA) has completed enrollment, with topline results expected in early 2026.
- Fanregratinib in China: Recruitment for the registrational Phase II study in patients with advanced intrahepatic cholangiocarcinoma (IHCC) in China has been completed, with new drug application submission preparation underway for the first half of 2026.
For more information on the R&D updates presentation, please visit www.hutch-med.com/event/.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including but not limited to its expectations regarding the therapeutic potential of HMPL‑A251 or other drug candidates from the ATTC platform, fanregratinib, fruquintinib, savolitinib and surufatinib, the further clinical development for HMPL‑A251 or other drug candidates from the ATTC platform, fanregratinib, fruquintinib, savolitinib and surufatinib , its expectations as to whether any studies on HMPL‑A251 or other drug candidates from the ATTC platform, fanregratinib, fruquintinib, savolitinib and surufatinib would meet their primary or secondary endpoints, and its expectations as to the timing of the completion and the release of results from such studies. Such risks and uncertainties include, among other things, assumptions regarding enrollment rates and the timing and availability of subjects meeting a study’s inclusion and exclusion criteria; changes to clinical protocols or regulatory requirements; unexpected adverse events or safety issues; the ability of HMPL‑A251 or other drug candidates from the ATTC platform, fanregratinib, fruquintinib, savolitinib and surufatinib, including as combination therapies, to meet the primary or secondary endpoint of a study, to obtain regulatory approval in different jurisdictions and to gain commercial acceptance after obtaining regulatory approval; the potential markets of HMPL‑A251 or other drug candidates from the ATTC platform, fanregratinib, fruquintinib, savolitinib and surufatinib for a targeted indication, and the sufficiency of funding. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
| ➤ Chinese (Putonghua) Session: In-person* and Online webcast |
| October 31, 2025 (Friday) |
| 3pm HKT / 3am EDT / 7am GMT |
| * The in-person event is by invitation only. |
| ➤ English Session: Online Webcast |
| October 31, 2025 (Friday) |
| 8pm HKT / 8am EDT / 12 noon GMT |
— First investigational drug candidate using the HUTCHMED ATTC technology platform to create potent targeted therapy payloads while mitigating related toxicities —
— Unique, highly potent PI3K/PIKK inhibitor payload optimized to exploit antibody-conjugate advantages, with directed delivery and low plasma exposure of free payload —
— Preclinical data shows robust antitumor activity with synergistic and bystander killing effects —
Hong Kong, Shanghai & Florham Park, NJ — Thursday, October 23, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces preclinical data for HMPL-A251 at the AACR‑NCI‑EORTC International Conference on Molecular Targets and Cancer Therapeutics, held October 22–26, 2025, in Boston, USA. HMPL-A251 is a first-in-class PI3K/AKT/mTOR (“PAM”)-HER2 Antibody-Targeted Therapy Conjugate (“ATTC”) comprising of a highly selective and potent PI3K/PIKK inhibitor payload linked to a humanized anti-HER2 IgG1 antibody, via a cleavable linker.
HER2 is a well-established therapeutic target. HER2 overexpression is found in a variety of cancer types and often associated with poor prognosis. As a key downstream signaling pathway of HER2, the PAM pathway contributes significantly to the resistance against HER2-targeting treatments when altered. HMPL-A251 is innovatively designed to leverage the synergy between HER2 targeting and PAM pathway inhibition to address limitations of traditional toxin-based antibody-drug conjugates (“ADCs”) and standalone PAM inhibitors.
In vitro, the PI3K/PIKK inhibitor payload exhibited high potency, selectivity, and broad anti-tumor activity across a panel of 130 tumor cell lines. By conjugating this potent payload with an anti-HER2 antibody via a hydrophilic linker, the ATTC compound HMPL-A251, upon binding to the HER2-positive target cells, undergoes rapid internalization, lysosomal trafficking, payload release, and inhibition of PAM and PIKK signaling, inducing tumor cell apoptosis. HMPL-A251 demonstrated HER2-dependent antitumor activity in vitro, potently inhibiting HER2-positive tumor cell growth regardless of PAM pathway alterations, with moderately reduced activity in HER2-low, PAM-altered cell lines. HMPL-A251 also demonstrated a bystander effect on HER2-null cells when co-cultured with HER2-positive cells.
Unlike toxin-based ADCs, which often face challenges with toxicity related to their cytotoxic payloads, ATTCs are designed to prioritize tumor-specific delivery of a pathway-modulating payload, enhancing safety for long‑term use and enabling potential frontline combinations with chemotherapy. In vivo, HMPL-A251 demonstrated superior anti-tumor efficacy and tolerability as compared to the naked antibody and payload administered together. A single intravenous dose of HMPL-A251 induced tumor regression across multiple models including HER2-positive and HER2-low models with or without PAM alteration. Efficacy correlated strongly with payload concentration and target inhibition in tumor tissue. Notably, when benchmarked against T-DXd (trastuzumab deruxtecan, a HER2‑directed ADC), HMPL-A251 achieved superior or comparable efficacy at equivalent doses in most tested models. Moreover, payload-based toxicities are expected to be low, as the plasma exposure of free payload was much lower than for HMPL-251, with a mass ratio of less than 1:500,000.
“We are excited to share the progress of HMPL-A251, the first candidate from our ATTC platform. It represents a potentially significant leap forward in addressing the limitations of toxin-based ADCs and narrow therapeutic window of systemic PAM inhibitors. By combining selective PI3K/PIKK inhibition with precise HER2 targeting, HMPL-A251 achieves potent antitumor effects while maintaining a favorable safety profile,” said Dr Michael Shi, Head of R&D and Chief Medical Officer of HUTCHMED. “The compelling preclinical data presented underscore its potential to redefine treatment for a wide spectrum of cancers, and we are excited to advance HMPL-A251 as well as more ATTC drug candidates toward clinical trials.”
HUTCHMED plans to initiate global clinical trials for HMPL-A251 around the end of 2025, followed by multiple global Investigational New Drug (IND) filings for more ATTC candidates in 2026.
About the ATTC platform
HUTCHMED’s Antibody-Targeted Therapy Conjugate platform represents a next-generation approach to precision oncology, combining monoclonal antibodies with proprietary small-molecule inhibitor payloads to deliver dual mechanisms of action. Unlike traditional cytotoxin-based ADCs, ATTCs combine targeted therapies to achieve synergistic anti-tumor activity and durable responses in preclinical models, outperforming standalone antibody or small-molecule inhibitor components in efficacy and safety.
Built on over 20 years of targeted therapy expertise, the platform enables development of drug candidates for diverse cancer types. By leveraging antibody-guided delivery and tumor-specific payload release, ATTCs improve the accessibility to tumors and reduce off-tumor toxicity. This overcomes challenges of traditional small-molecule inhibitors, ensures safer long-term use, and supports combinations with chemotherapy and immunotherapy, unlocking potential for early-line treatments.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including but not limited to its expectations regarding the therapeutic potential of HMPL‑A251 and other drug candidates from the ATTC platform, the further clinical development for HMPL‑A251 and other drug candidates from the ATTC platform, its expectations as to whether any studies on HMPL‑A251 and other drug candidates from the ATTC platform would meet their primary or secondary endpoints, and its expectations as to the timing of the completion and the release of results from such studies. Such risks and uncertainties include, among other things, assumptions regarding enrollment rates and the timing and availability of subjects meeting a study’s inclusion and exclusion criteria; changes to clinical protocols or regulatory requirements; unexpected adverse events or safety issues; the ability of HMPL‑A251 and other drug candidates from the ATTC platform, including as combination therapies, to meet the primary or secondary endpoint of a study, to obtain regulatory approval in different jurisdictions and to gain commercial acceptance after obtaining regulatory approval; the potential markets of HMPL‑A251 other drug candidates from the ATTC platform for a targeted indication, and the sufficiency of funding. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Hong Kong, Shanghai & Florham Park, NJ — Tuesday, October 21, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM: HCM; SEHK:13) announces that the non-performance based awards granted under the Long Term Incentive Plan (“LTIP”) on October 20, 2021 to the following persons discharging managerial responsibilities were vested on October 20, 2025:-
| Award Holder | Number of American depositary shares (“ADS”) | |
| Dr Dan Eldar (Non-executive Director (“NED”)) | 1,938 | |
| Ms Edith Shih (NED) | 1,938 (1) | |
| Professor Tony Mok (Independent Non-executive Director) | 1,938 |
Note:
(1) These ADSs were not received by Ms Edith Shih, but were received by or for the account of her employer, Hutchison International Limited.
The notifications set out below are provided in accordance with the requirements of the UK Market Abuse Regulation.
(a) Dr Dan Eldar
| 1 | Details of the person discharging managerial responsibilities/person closely associated | |||||
| a) | Name | Dr Dan Eldar | ||||
| 2 | Reason for the notification | |||||
| a) | Position/status | Non-Executive Director | ||||
| b) | Initial notification/Amendment | Initial notification | ||||
| 3 | Details of the issuer, emission allowance market participant, auction platform, auctioneer or auction monitor | |||||
| a) | Name | HUTCHMED (China) Limited | ||||
| b) | LEI | 2138006X34YDQ6OBYE79 | ||||
| 4 | Details of the transaction(s): section to be repeated for (i) each type of instrument; (ii) each type of transaction; (iii) each date; and (iv) each place where transactions have been conducted | |||||
| a) | Description of the financial instrument, type of instrument Identification code |
ADS each representing five Ordinary Shares of US$0.10 ADS ISIN: US44842L1035 |
||||
| b) | Nature of the transaction | Vesting of awards granted on October 20, 2021 under HUTCHMED’s LTIP | ||||
| c) | Price(s) and volume(s) |
|
||||
| d) | Aggregated information
|
N/A | ||||
| e) | Date of the transaction | 2025-10-20 | ||||
| f) | Place of the transaction | Outside a trading venue | ||||
(b) Professor Tony Mok
| 1 | Details of the person discharging managerial responsibilities/person closely associated | |||||
| a) | Name | Professor Tony Mok | ||||
| 2 | Reason for the notification | |||||
| a) | Position/status | Independent Non-Executive Director | ||||
| b) | Initial notification/Amendment | Initial notification | ||||
| 3 | Details of the issuer, emission allowance market participant, auction platform, auctioneer or auction monitor | |||||
| a) | Name | HUTCHMED (China) Limited | ||||
| b) | LEI | 2138006X34YDQ6OBYE79 | ||||
| 4 | Details of the transaction(s): section to be repeated for (i) each type of instrument; (ii) each type of transaction; (iii) each date; and (iv) each place where transactions have been conducted | |||||
| a) | Description of the financial instrument, type of instrument Identification code |
ADS each representing five Ordinary Shares of US$0.10 ADS ISIN: US44842L1035 |
||||
| b) | Nature of the transaction | Vesting of awards granted on October 20, 2021 under HUTCHMED’s LTIP | ||||
| c) | Price(s) and volume(s) |
|
||||
| d) | Aggregated information
|
N/A | ||||
| e) | Date of the transaction | 2025-10-20 | ||||
| f) | Place of the transaction | Outside a trading venue | ||||
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
The Board of Directors of HUTCHMED (China) Limited comprises 9 Directors as follows:
Chairman and Non-executive Director
Dr Dan ELDAR
Executive Directors
Dr Weiguo SU (Chief Executive Officer* and Chief Scientific Officer)
Mr CHENG Chig Fung, Johnny (Acting Chief Executive Officer and Chief Financial Officer)
Non-executive Directors
Ms Edith SHIH
Ms Ling YANG
Independent Non-executive Directors
Professor MOK Shu Kam, Tony (Senior and Lead Independent Non-executive Director)
Dr Renu BHATIA
Dr Chaohong HU
Professor TAN Shao Weng, Daniel
Mr WONG Tak Wai
The table below provides membership information of the committees on which Board members serve.
| Board Committee | |||||
|---|---|---|---|---|---|
| Directors | Audit Committee | Nomination Committee | Remuneration Committee | Sustainability Committee | Technical Committee |
| Dan ELDAR | – | M | – | – | M |
| Weiguo SU | – | – | – | – | M |
| CHENG Chig Fung, Johnny | – | – | – | M | – |
| Edith SHIH | – | – | M | C | – |
| Ling YANG | – | – | – | – | – |
| MOK Shu Kam, Tony | – | C | – | M | C |
| Renu BHATIA | M | – | C | – | M |
| Chaohong HU | M | M | – | – | M |
| TAN Shao Weng, Daniel | – | – | – | – | M |
| WONG Tak Wai | C | – | M | – | – |
Notes:
* On leave from duties
C Chairman of the relevant Board committees
M Member of the relevant Board committees
October 15, 2025
Hong Kong, Shanghai & Florham Park, NJ — Tuesday, October 14, 2025: HUTCHMED (China) Limited (“HUTCHMED” or the “Company”) (Nasdaq/AIM:HCM, HKEX:13) today announces that Professor Tan Shao Weng, Daniel (Chen Shaowen) is appointed as an Independent Non-executive Director and a member of the Technical Committee of the Company with effect from October 15, 2025.
Professor Tan, aged 47, has over 20 years of experience in oncology, with his main research interests focusing on thoracic, head and neck malignancies, and drug development. He is the head of the Division of Clinical Trials and Epidemiological Sciences and a senior consultant in the Division of Medical Oncology at the National Cancer Centre Singapore (“NCCS”). He is a professor at Duke-NUS Medical School and serves as a senior clinician-scientist at the Genome Institute of Singapore.
Dr Dan Eldar, Chairman of HUTCHMED, said “On behalf of the Board, I would like to extend a warm welcome to Professor Tan. Professor Tan has a proven track record in early phase clinical trials of novel therapeutics, and we believe that his expertise in targeted therapies, biomarker development and clinical oncology will strongly support the strategic growth of the Company and drive continued innovation in cancer drug development.”
Professor Tan currently leads the Experimental Cancer Therapeutics Unit (ECRU) at the NCCS, one of Asia’s largest Phase I units, which runs approximately 30–40 trials at any time. He is also the Principal Investigator of the Cancer Therapeutics Research Laboratory at NCCS focused on developing representative patient-derived preclinical models to study drug response and resistance, complementing his leadership in multiple biomarker-driven early-phase and first-in-human clinical trials. He currently serves as the principal investigator of the National Medical Research Council (“NMRC”) Lung Cancer Large Collaborative Grant in Singapore (2025-2029 and previously 2019–2023) and chairs the Asian Thoracic Oncology Research Group, a regional platform for translational research and clinical trials.
Professor Tan’s research focuses on applying “omics” technologies to understand drug resistance and accelerate the development of novel therapies and biomarkers. His work has earned numerous accolades, including the International Association for the Study of Lung Cancer (IASLC) Daniel C. Idhe Lectureship Award for Medical Oncology, the American Society of Clinical Oncology (ASCO) Young Investigator Award, and the SingHealth Group Chief Executive Officer Outstanding Clinician-Researcher Award. He is a three-time recipient of the NMRC Clinician-Scientist Award.
He has published extensively, contributing to over 200 peer-reviewed articles including those in leading journals such as Nature, Nature Medicine, New England Journal of Medicine, and The Lancet Oncology. Professor Tan has held leadership roles in global oncology forums, including Chair of the IASLC Education Committee and Co-Chair of the World Conference on Lung Cancer (WCLC). He currently serves as Associate Editor for the Journal of Thoracic Oncology, Scientific Editor for Cancer Discovery and Senior Editor for Clinical Cancer Research. He is a member of the American Association of Cancer Research (AACR), ASCO, the European Society for Medical Oncology (ESMO), IASLC and the Singapore Society of Oncology of which he was also a past secretary.
Professor Tan holds a Bachelor of Science degree with first class honors in tumor biology from University College London, University of London; a Bachelor of Medicine and Bachelor of Surgery from St Bartholomew’s and the Royal London School of Medicine and Dentistry; and a PhD from the National University of Singapore. He is a member of the Royal College of Physicians of the United Kingdom.
Additional Disclosure Pursuant to the AІM Rules for Companies and the Rules Governing the Listing of Securities on The Stock Exchange of Hong Kong Limited (“HK Listing Rules”)
Professor Tan is currently a director of Epoch Biosciences Pte. Ltd. Save for this appointment, Professor Tan has held no other directorships or partnerships during the period of five years prior to his appointment as a director of HUTCHMED. He does not have any relationship with any Directors, senior management or substantial or controlling shareholders of HUTCHMED. Professor Tan has confirmed (a) his independence as regards each of the factors for independence referred to in Rule 3.13(1) to (8) of the HK Listing Rules; (b) that he had no past or present financial or other interest in the business of the Company or its subsidiaries or any connection with any core connected person (as defined in the HK Listing Rules) of the Company; and (c) that there are no other factors that may affect his independence at the time of his appointment.
The initial term of the appointment of Professor Tan as an Independent Non-executive Director of the Company shall end at the next following annual general meeting of the Company, subject to retirement in accordance with the Articles of Association of the Company and applicable legal and regulatory requirements, and thereafter for successive periods of 12 months, unless he is not re-elected at the next following annual general meeting or his appointment is otherwise terminated earlier by either party in writing. The director’s fees of Professor Tan as an Independent Non-executive Director and member of the Technical Committee of the Company under his appointment letter are US$76,000 and US$8,000 per annum respectively, which were determined by the Board with reference to the director’s duties and responsibilities and prevailing market conditions. Such fees are subject to review from time to time and proration for any incomplete year of service.
Professor Tan does not have any interest in any shares of the Company within the meaning of Part XV of the Securities and Futures Ordinance (Cap. 571 of the Laws of Hong Kong).
Save for the information disclosed above, there is no other information in relation to Professor Tan that is required to be disclosed pursuant to Rule 17 and Schedule 2(g) of the AІM Rules for Companies or Rule 13.51(2) of the HK Listing Rules, and there are no other matters concerning the appointment of Professor Tan that are required to be brought to the attention of the shareholders of HUTCHMED.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery, global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception, HUTCHMED has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit www.hutch-med.com or follow us on LinkedIn.
Forward-Looking Statements
This announcement contains forward-looking statements within the meaning of the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, the risk that current or future appointees to HUTCHMED’s board of directors are not effective in their respective positions, the difficulty in locating and recruiting suitable candidates for its board of directors and the management difficulties which may arise from changes in HUTCHMED’s board of directors. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the U.S. Securities and Exchange Commission, on AIM and with The Stock Exchange of Hong Kong Limited. HUTCHMED undertakes no obligation to update or revise the information contained in this announcement, whether as a result of new information, future events or circumstances or otherwise.
— The fruquintinib and sintilimab combination demonstrated significant PFS improvements in advanced renal cell carcinoma patients after progression on first-line therapies —
Hong Kong, Shanghai & Florham Park, NJ — Monday, October 13, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) announces results from the FRUSICA-2 registration clinical trial of the fruquintinib and sintilimab combination for the treatment of patients with locally advanced or metastatic renal cell carcinoma. Results of the Phase III part of the study will be presented on Friday, October 17, 2025 during the European Society for Medical Oncology (“ESMO”) Congress in Berlin, Germany.
FRUSICA-2 is a randomized, open-label, active-controlled registration study evaluating the efficacy and safety of fruquintinib in combination with sintilimab versus axitinib or everolimus monotherapy for the second-line treatment of advanced renal cell carcinoma (NCT05522231). A total of 234 patients were randomized into a group that received fruquintinib plus sintilimab combination therapy, or into a group that received axitinib or everolimus monotherapy. As of the progression free survival (“PFS”) final analysis cutoff of February 17, 2025, the median follow-up was 16.6 months.
The median PFS as assessed by blinded independent central review (BICR) was 22.2 months with fruquintinib plus sintilimab, compared to 6.9 months with axitinib/everolimus (stratified hazard ratio [HR] 0.373; stratified log-rank p<0.0001). The objective response rate (ORR) was 60.5% vs 24.3% (Odds Ratio 4.622, p<0.0001), and the median duration of response (DoR) was 23.7 vs 11.3 months, respectively. Overall survival data were still evolving at the time of data cutoff with maturity of approximately 20%. Efficacy benefits were observed in all prognostic risk groups, as defined by the International mRCC Database Consortium (IMDC) criteria.
The safety profile of the fruquintinib and sintilimab combination was tolerable and consistent with the known profiles of each individual treatment. Treatment-emergent adverse events (TEAEs) of grade 3 or above occurred in 71.4% of patients in the fruquintinib plus sintilimab group compared to 58.8% for patients in the axitinib/everolimus group.
“The FRUSICA-2 trial results provide compelling evidence that fruquintinib and sintilimab may offer a valuable new treatment option for patients with advanced renal cell carcinoma,” said Professor Dingwei Ye of Fudan University Shanghai Cancer Center and the co-leading Principal Investigator of the FRUSICA-2 study. “These findings show the combination’s potential to address a critical unmet need for this patient population, delivering consistent benefits across varied patient profiles and prognostic risk groups.”
“The FRUSICA-2 study suggests that fruquintinib and sintilimab could play a meaningful role in shaping second-line treatment strategies for advanced renal cell carcinoma,” said Professor Zhisong He of Peking University First Hospital and the co-leading Principal Investigator of the FRUSICA-2 study. “These results point to the combination’s potential to enhance clinical outcomes, providing a new option for managing this challenging disease.”
Supported by data from FRUSICA-2, a New Drug Application (NDA) for the combination of fruquintinib and sintilimab in patients with locally advanced or metastatic renal cell carcinoma who have failed prior treatment has been accepted for review by the China National Medical Products Administration (NMPA).
About Kidney Cancer and Renal Cell Carcinoma
It is estimated that approximately 435,000 new patients were diagnosed with kidney cancer worldwide in 2022.[1] In China, an estimated 74,000 new patients were diagnosed with kidney cancer in 2022.[2] Approximately 90% of kidney tumors are renal cell carcinoma.
The safety and efficacy of fruquintinib for the investigational uses discussed above have not been established and there is no guarantee that it will receive health authority approval or become commercially available in any country for the uses being investigated.
About Fruquintinib
Fruquintinib is a selective oral inhibitor of all three vascular endothelial growth factor receptors (“VEGFR”) -1, -2 and -3. VEGFR inhibitors play a pivotal role in inhibiting tumor angiogenesis. Fruquintinib was designed to limit off-target kinase activity and improve drug exposure to achieve sustained target inhibition.[3]
Fruquintinib is co-developed and co-commercialized in China by HUTCHMED and Eli Lilly and Company under the brand name ELUNATE®. It is approved for the treatment of patients with metastatic colorectal cancer who have previously received fluoropyrimidine, oxaliplatin and irinotecan-based chemotherapy, and those who have previously received or are not suitable to receive anti-VEGF therapy or anti-epidermal growth factor receptor (EGFR) therapy (RAS wild-type) in China. The combination of fruquintinib and sintilimab has received conditional approval in China for the treatment of patients with advanced pMMR endometrial cancer who have failed prior systemic therapy and are not candidates for curative surgery or radiation.
Takeda holds the exclusive worldwide license to further develop, commercialize, and manufacture fruquintinib outside mainland China, Hong Kong and Macau, marketing it under the brand name FRUZAQLA®.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including but not limited to its expectations regarding the therapeutic potential of fruquintinib, the further clinical development for fruquintinib, its expectations as to whether any studies on fruquintinib would meet their primary or secondary endpoints, and its expectations as to the timing of the completion and the release of results from such studies. Such risks and uncertainties include, among other things, assumptions regarding enrollment rates and the timing and availability of subjects meeting a study’s inclusion and exclusion criteria; changes to clinical protocols or regulatory requirements; unexpected adverse events or safety issues; the ability of fruquintinib, including as combination therapies, to meet the primary or secondary endpoint of a study, to obtain regulatory approval in different jurisdictions and to gain commercial acceptance after obtaining regulatory approval; the potential markets of fruquintinib for a targeted indication, and the sufficiency of funding. In addition, as certain studies rely on the use of other drug products such as sintilimab as combination therapeutics, such risks and uncertainties include assumptions regarding their safety, efficacy, supply and continued regulatory approval. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
References
[1] The Global Cancer Observatory, kidney cancer fact sheet. https://gco.iarc.who.int/media/globocan/factsheets/cancers/29-kidney-fact-sheet.pdf. Accessed February 19, 2025.
[2] The Global Cancer Observatory, China fact sheet. https://gco.iarc.who.int/media/globocan/factsheets/populations/160-china-fact-sheet.pdf. Accessed February 19, 2025.
[3] Sun Q, et al. Discovery of fruquintinib, a potent and highly selective small molecule inhibitor of VEGFR 1, 2, 3 tyrosine kinases for cancer therapy. Cancer Biol Ther. 2014;15(12):1635-45. doi: 10.4161/15384047.2014.964087.
Hong Kong, Shanghai & Florham Park, NJ — Thursday, October 2, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that new and updated data from several studies of compounds discovered by HUTCHMED will be presented at the European Society for Medical Oncology (“ESMO”) Congress 2025, taking place on October 17-21, 2025 in Berlin, Germany.
Results from the FRUSICA-2 registration study of the fruquintinib and sintilimab combination as a second-line treatment for locally advanced or metastatic renal cell carcinoma will be presented in a Mini Oral session. Additionally, further analyses of the fruquintinib FRUSICA-1 study in endometrial cancer and the savolitinib SACHI and SAVANNAH studies in non-small cell lung cancer will be presented during the poster sessions.
Details of the presentations are as follows:
Abstract titlePresenter / Lead authorPresentation details
SPONSORED STUDIES |
|||
| Fruquintinib (FRUQ) plus sintilimab (SIN) versus axitinib (AXI) or everolimus (EVE) monotherapy as 2L treatment in pts with locally advanced or metastatic renal cell carcinoma (RCC): results from phase 3 part of a randomized, open-label, active-controlled phase 2/3 study (FRUSICA-2) | Zhenhua Liu (Chengdu, China) |
2592MO Mini Oral Session 1: GU tumours, renal & urothelial Friday, Oct 17, 2025 Karlsruhe Auditorium – Hall 5.2 16:00 – 17:30 CEST |
|
| A Fruquintinib Expanded Access Program (EAP) to Provide Treatment for Patients With Metastatic Colorectal Cancer (mCRC) | Stefan Kasper-Virchow (Essen, Germany) |
794P Poster Session: Colorectal cancer |
|
| Fruquintinib plus tislelizumab in microsatellite stable metastatic colorectal cancer: Results from a phase 1b/2 study | N. Arvind Dasari (Houston, USA) |
799P Poster Session: Colorectal cancer |
|
| A novel artificial intelligence (AI) imaging biomarker of tumor vascularity and heterogeneity radiomics to predict survival benefit of fruquintinib vs placebo in metastatic colorectal cancer (mCRC) | Sara Lonardi (Padua, Italy) |
804P Poster Session: Colorectal cancer |
|
| Safety and tolerability of fruquintinib: Pooled analysis of three placebo-controlled studies in patients with metastatic colorectal cancer | Cathy Eng (Nashville, USA) |
811P Poster Session: Colorectal cancer |
|
| Association between Metabolic Syndrome (MetS) and clinical outcomes of Fruquintinib plus Sintilimab in Previously Treated Advanced Endometrial Cancer (EMC) Patients with pMMR Status: results from FRUSICA-1 study | Danbo Wang (Shenyang, China) |
1230eP Poster Session: Gynaecological Cancer |
|
| ctDNA analysis in phase 3 SACHI trial: savolitinib (savo) plus osimertinib (osi) versus chemotherapy (chemo) in MET-amplified (METamp) advanced NSCLC after disease progression (PD) on EGFR tyrosine kinase inhibitor (TKI) | Yongfeng Yu (Shanghai, China) |
1954P Poster Session: NSCLC, metastatic |
|
| SAVANNAH: Safety and tolerability of osimertinib (osi) + savolitinib (savo) in EGFRm advanced NSCLC with MET overexpression and/or amplification (OverExp/Amp) following disease progression on osi | Quincy Siu-chung Chu (Edmonton, Canada) |
1955P Poster Session: NSCLC, metastatic |
|
| MET testing and treatment (tx) sequencing after progression on first line (1L) osimertinib (osi) in patients (pts) with EGFRm advanced NSCLC and acquired MET overexpression and/or amplification (OverExp/Amp): interim analysis of a global real world (rw) study | Julia Rotow (Boston, USA) |
1956P Poster Session: NSCLC, metastatic |
|
| INVESTIGATOR-INITIATED STUDIES |
|||
| Fruquintinib plus sintilimab and SOX as conversion therapy for initially unresectable gastric/gastroesophageal junction adenocarcinoma (GC/GEJC): Updated surgical and survival results from the single-arm, phase 2 clinical trial | Fei Ma (Zhengzhou, China) |
2159P Poster Session: Oesophagogastric cancer |
|
| Fruquintinib alternating with bevacizumab plus capecitabine as maintenance therapy after first-line treatment in metastatic colorectal cancer (mCRC): A multicenter, open-label, Phase II Study | Wangjun Liao (Guangzhou, China) |
898eP E-poster Session: Colorectal cancer |
|
| The efficacy and safety of surufatinib combined with chemotherapy in the first-line treatment of advanced periampullary carcinoma: a single arm, prospective, exploratory clinical study | Qianqian Wang (Nanjing, China) |
929P Poster Session: Developmental therapeutics |
|
| Surufatinib-Based Late-Line Therapy Outcomes in Recurrent Metastatic NSCLC: Monotherapy and Vinorelbine Combination Regimens | Yanfang Zheng (Guangzhou, China) |
1884P Poster Session: NSCLC, metastatic |
|
| Surufatinib combined with Toripalimab, Pemetrexed, and Platinum in Advanced Non-Squamous Non-Small Cell Lung Cancer (nsg-NSCLC): Final Phase ll Results from a Single-Center Trial | Wenfeng Fang/ Li Zhang (Guangzhou, China) |
1887P Poster Session: NSCLC, metastatic |
|
| Efficacy/safety and preliminary scRNA-seq results of surufatinib plus gemcitabine and nab-paclitaxel as neoadjuvant therapy in resectable and borderline resectable pancreatic cancer | Song Gao/ Jihui Hao (Tianjin, China) |
2236P Poster Session: Pancreatic cancer |
|
| Efficacy and Safety of Surufatinib in Patients with Advanced Soft Tissue Sarcoma After Failure of Anthracycline Chemotherapy and Prior Effective Antiangiogenic Therapy: A Single-Arm, Prospective, Exploratory Phase II Study | Xiaowei Zhang/ Zhiguo Luo (Shanghai, China) | 2716P Poster Session: Sarcoma |
|
About Fruquintinib
Fruquintinib is a selective oral inhibitor of all three vascular endothelial growth factor receptors (“VEGFR”) -1, ‑2 and -3. Fruquintinib is co-developed and co-commercialized in China by HUTCHMED and Eli Lilly and Company under the brand name ELUNATE®. Takeda holds the exclusive worldwide license to further develop, commercialize, and manufacture fruquintinib outside mainland China, Hong Kong and Macau, marketing it under the brand name FRUZAQLA®.
About Savolitinib
Savolitinib is an oral, potent and highly selective MET tyrosine kinase inhibitor that has demonstrated clinical activity in advanced solid tumors. It blocks atypical activation of the MET receptor tyrosine kinase pathway that occurs because of mutations (such as exon 14 skipping alterations or other point mutations), gene amplification or protein overexpression. Savolitinib is being jointly developed by AstraZeneca and HUTCHMED, and commercialized by AstraZeneca under the brand name ORPATHYS®.
About Surufatinib
Surufatinib is a novel, oral angio-immuno kinase inhibitor that selectively inhibits the tyrosine kinase activity associated with VEGFRs and fibroblast growth factor receptor (FGFR), which both inhibit angiogenesis, and colony stimulating factor-1 receptor (CSF-1R), which regulates tumor-associated macrophages, promoting the body’s immune response against tumor cells. Surufatinib is marketed in China by HUTCHMED under the brand name SULANDA®. HUTCHMED currently retains all rights to surufatinib worldwide.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including but not limited to its expectations regarding the therapeutic potential of fruquintinib, surufatinib and savolitinib, the further clinical development for fruquintinib, surufatinib and savolitinib, its expectations as to whether any studies on fruquintinib, surufatinib and savolitinib would meet their primary or secondary endpoints, and its expectations as to the timing of the completion and the release of results from such studies. Such risks and uncertainties include, among other things, assumptions regarding enrollment rates and the timing and availability of subjects meeting a study’s inclusion and exclusion criteria; changes to clinical protocols or regulatory requirements; unexpected adverse events or safety issues; the ability of fruquintinib, surufatinib and savolitinib, including as combination therapies, to meet the primary or secondary endpoint of a study, to obtain regulatory approval in different jurisdictions and to gain commercial acceptance after obtaining regulatory approval; the potential markets of fruquintinib, surufatinib and savolitinib for a targeted indication, and the sufficiency of funding. In addition, as certain studies rely on the use of other drug products such as sintilimab and toripalimab as combination therapeutics, such risks and uncertainties include assumptions regarding their safety, efficacy, supply and continued regulatory approval. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
— HUTCHMED will host in-person presentation and online webinar on Friday, October 31 —
Hong Kong, Shanghai & Florham Park, NJ — Friday, September 12, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that it will host a Research & Development update in Shanghai, China, and via webcast on Friday, October 31, 2025.
During the event, Dr. Michael Shi, Executive Vice President, Head of R&D and Chief Medical Officer of HUTCHMED, will share insights into the Company’s R&D strategy and vision. This will include an overview of the Company’s Antibody Targeted Therapy Conjugates platform, featuring its lead candidate HMPL-A251, as well as updates on late-stage pipeline development.
The in-person event will be held in Shanghai from 3:00 p.m. to 5:00 p.m. HKT and conducted in Chinese (Putonghua). A live webcast will be available concurrently. Attendance at the in-person event is by invitation only.
A webcast in English will be held from 8:00 p.m. HKT / 8:00 a.m. EDT / 12:00 noon GMT on Friday, October 31, for approximately one hour.
Both webcasts will be available live via the company website at www.hutch-med.com/event/. A replay will also be available on the website shortly after the event.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements involve risks and uncertainties. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the U.S. Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Hong Kong, Shanghai & Florham Park, NJ — Friday, September 5, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that new and updated data from several studies of compounds discovered by HUTCHMED will be presented at the 2025 World Conference on Lung Cancer (“WCLC”) taking place on September 6-9, 2025 in Barcelona, Spain, and the Chinese Society of Clinical Oncology (“CSCO”) Annual Meeting 2025, taking place on September 10-14, 2025 in Jinan, China.
Updated analysis from savolitinib’s SACHI, SAVANNAH and a Phase IIIb confirmatory study in non-small cell lung cancer (“NSCLC”) patients will be presented at WCLC 2025. Savolitinib is an oral, potent and highly selective MET tyrosine kinase inhibitor (“TKI”) being jointly developed by AstraZeneca and HUTCHMED and commercialized by AstraZeneca. Details of the WCLC 2025 presentations are as follows:
| Abstract title | Presenter / Lead author | Presentation details | |
SPONSORED STUDIES |
|||
| SAVANNAH: Biomarker Concordance and Acquired Resistance in Patients with EGFRm MET-OverExp and / or Amp NSCLC |
Christina Baik, University of Washington and Fred Hutchinson Cancer Center, Seattle, USA | MA03.03 Mini Oral: New Advances in Circulating Biomarkers Room 06 Sunday, September 7, 2025 3:15 – 4:30PM CEST |
|
| Efficacy and Safety of Savolitinib in Advanced or Metastatic METex14 NSCLC Patients With or Without Prior Immunotherapy |
Yongfeng Yu, Shanghai Chest Hospital, Shanghai, China | P3.12.48 Poster: Metastatic NSCLC – Targeted Therapy Tuesday, September 9, 2025 |
|
| Frontline Treatment Duration in MET-Amplified NSCLC After Third-Generation EGFR-TKI Failure: SACHI Study Insights |
Lijuan Chen, Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, China | P3.12.64 Poster: Metastatic NSCLC – Targeted Therapy Tuesday, September 9, 2025 |
|
| Osimertinib + Savolitinib in EGFRm Advanced NSCLC With MET Overexp And/Or Amp Post-Progression on Osimertinib: SAVANNAH PROs |
Silvia Novello, University of Turin, San Luigi Hospital, Turin, Italy | PT2.12.04 ePoster: Metastatic NSCLC – Targeted Therapy Monday, September 8, 2025 |
|
| INVESTIGATOR-INITIATED STUDIES |
|||
| Efficacy and Safety of Surufatinib, Durvalumab in Combined with Chemotherapy as First-line Treatment of Extensive-stage Small-cell Lung Cancer | Hui Zhang/ Ying Hu, Beijing Chest Hospital, Beijing, China | P3.13.22 Poster: Small Cell Lung Cancer and Neuroendocrine Tumors Tuesday, September 9, 2025 |
|
Clinical data of HMPL-653, a novel, selective and potent CSF-1R inhibitor, from a first-in-human Phase I study in patients with tenosynovial giant cell tumor in China will be presented for the first time at the CSCO Annual Meeting 2025. Details of the CSCO Annual Meeting 2025 presentations are as follows:
| Abstract title | Presenter / Lead author | Presentation details | |
SPONSORED STUDIES |
|||
| A first-in-human phase I study of HMPL-653, a CSF-1R inhibitor, in patients with tenosynovial giant cell tumor | Xiaohui Niu | 25297 Oral Session Friday, September 12, 2025 15:00 – 15:12PM HKT |
|
| INVESTIGATOR-INITIATED STUDIES |
|||
| Fruquintinib Plus Serplulimab as First-Line Therapy in Metastatic or Unresectable Non-Clear Cell Renal Cell Carcinoma (nccRCC): Updated Efficacy and Safety from a Multicenter, Single-Arm Trial | Jiwei Huang/ Wei Xue | 23258 Oral Session Thursday, September 11, 2025 16:50 – 17:15PM HKT |
|
| Fruquintinib plus camrelizumab combined with paclitaxel liposome and nedaplatin as first-line treatment for advanced esophageal squamous cell carcinoma (ESCC): updated data from a single-arm, phase II clinical trial | Tianzhu Qiu/ Yanhong Gu | 23766 Oral Session Friday, September 12, 2025 11:20 – 12:00 noon HKT |
|
| Fruquintinib plus chemotherapy as second-line therapy in metastatic colorectal cancer: a multicenter, open-label, phase II clinical trial | Yongshun Chen | 22084 Poster Session |
|
| Efficacy and Safety of Neoadjuvant Fruquintinib plus Toripalimab and Short-Course Radiotherapy (SCRT) for Locally Advanced Rectal Cancer: Updated Results from a Phase II Clinical Trial | Zhiping Li | 21915 Abstract |
|
| Fruquintinib combined with chemotherapy as first-line treatment for advanced metastatic colorectal cancer: a propensity score‑matched comparison of efficacy between a prospective single-arm cohort and a retrospective observational cohort | Fuxiang Zhou | 23550 Abstract |
|
| Efficacy and safety of fruquintinib combined with PD-1 inhibitor and chidamide in MSS mCRC: a comparison with real-world bevacizumab plus anti-pd-1 and chidamide arm | Miaomiao Gou | 23591 Abstract |
|
| Phase ll Clinical Study of Surufatinib Combined with Gemcitabine and Cisplatin Plus Durvalumab/Pembrolizumab Regimen in the Treatment of Advanced Biliary Tract Cancer | Miaomiao Gou | 23610 Oral Session Friday, September 12, 2025 16:53 – 16:59PM HKT |
|
| A single-arm, Phase Ib/II trial of surufatinib plus KN046 and gemcitabine and nab-paclitaxel as first-line treatment for unresectable advanced pancreatic cancer | Wenquan Wang/ Liang Liu | 23783 Oral Session Thursday, September 11, 2025 16:20 – 16:35PM HKT |
|
| Updated results of surufatinib plus transarterial embolization versus surufatinib monotherapy in neuroendocrine tumor with liver metastasis: a prospective, randomized, controlled trial | Dan Cao | 22652 Poster Session |
|
| Surufatinib in patients with soft tissue myeloma who have failed first-line standard chemotherapy or anlotinib: a multicenter, prospective, two-cohort, phase II clinical study | Yuhong Zhou/ Xi Guo | P80 Poster Session |
|
| Efficacy and Mechanistic Study of the NASCA Regimen (Surufatinib Combined with Camrelizumab, Nab-Paclitaxel, and S‑1) in Advanced Pancreatic Cancer Patients with Liver Metastasis | Guanghai Dai/ Ru Jia | 22309 Abstract |
|
| A Phase II, Single-Arm Study of Surufatinib Combined with Zimberelimab and Nab-Paclitaxel in Patients with Advanced Triple‑Negative Breast Cancer: Data Update | Caixia Wang | 23679 Abstract |
|
| Efficacy and safety of surufatinib combined with gemcitabine, cisplatin and immune checkpoint inhibitor for the treatment of unresectable locally advanced or metastatic intrahepatic cholangiocarcinoma | Xuetao Shi/ Jingtao Zhong | 24133 Abstract |
|
| Efficacy and Safety of Surufatinib in Patients with Neuroendocrine Neoplasms: A Multicenter Retrospective Study | Jiang Long | 24294 Abstract |
|
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including but not limited to its expectations regarding the therapeutic potential of fruquintinib, surufatinib, savolitinib and HMPL-653, the further clinical development for fruquintinib, surufatinib, savolitinib and HMPL-653, its expectations as to whether any studies on fruquintinib, surufatinib, savolitinib and HMPL-653 would meet their primary or secondary endpoints, and its expectations as to the timing of the completion and the release of results from such studies. Such risks and uncertainties include, among other things, assumptions regarding enrollment rates and the timing and availability of subjects meeting a study’s inclusion and exclusion criteria; changes to clinical protocols or regulatory requirements; unexpected adverse events or safety issues; the ability of fruquintinib, surufatinib, savolitinib and HMPL-653, including as combination therapies, to meet the primary or secondary endpoint of a study, to obtain regulatory approval in different jurisdictions and to gain commercial acceptance after obtaining regulatory approval; the potential markets of fruquintinib, surufatinib, savolitinib and HMPL-653 for a targeted indication, and the sufficiency of funding. In addition, as certain studies rely on the use of other drug products as combination therapeutics, such risks and uncertainties include assumptions regarding their safety, efficacy, supply and continued regulatory approval. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
The Board of Directors of HUTCHMED (China) Limited comprises 9 Directors as follows:
Chairman and Non-executive Director
Dr Dan ELDAR
Executive Directors
Dr Weiguo SU (Chief Executive Officer* and Chief Scientific Officer)
Mr CHENG Chig Fung, Johnny (Acting Chief Executive Officer and Chief Financial Officer)
Non-executive Directors
Ms Edith SHIH
Ms Ling YANG
Independent Non-executive Directors
Professor MOK Shu Kam, Tony (Senior and Lead Independent Non-executive Director)
Dr Renu BHATIA
Dr Chaohong HU
Mr WONG Tak Wai
The table below provides membership information of the committees on which Board members serve.
| Board Committee | |||||
|---|---|---|---|---|---|
| Directors | Audit Committee | Nomination Committee | Remuneration Committee | Sustainability Committee | Technical Committee |
| Dan ELDAR | – | M | – | – | M |
| Weiguo SU | – | – | – | – | M |
| CHENG Chig Fung, Johnny | – | – | – | M | – |
| Edith SHIH | – | – | M | C | – |
| Ling YANG | – | – | – | – | – |
| MOK Shu Kam, Tony | – | C | – | M | C |
| Renu BHATIA | M | – | C | – | M |
| Chaohong HU | M | M | – | – | M |
| WONG Tak Wai | C | – | M | – | – |
Notes:
* On leave from duties
C Chairman of the relevant Board committees
M Member of the relevant Board committees
August 25, 2025
Hong Kong, Shanghai & Florham Park, NJ — Monday, August 25, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that Dr Weiguo Su, an Executive Director of the Company, will take a leave of absence from his duties as Chief Executive Officer due to health reasons. In light of this, the Board of Directors has appointed Mr Johnny Cheng, an Executive Director and Chief Financial Officer of the Company, as Acting Chief Executive Officer with immediate effect, in addition to his role as Chief Financial Officer.
Dr Dan Eldar, Chairman and Non-executive Director, said, “The Board expresses its full support for Dr Su and wishes him a speedy recovery. We thank Mr Cheng for his agreement to assume responsibility for overseeing the day-to-day operations and management of the Company during this interim period. The board has full confidence in Mr. Cheng’s capabilities to lead the Company. The Board is confident that all research, development and commercial initiatives will remain on track.”
Dr Weiguo Su, Executive Director, said, “This has been a very difficult decision to make, but at this time my focus must be on my health. I am certain that the Board, Mr Cheng and everyone at HUTCHMED will ensure the continued execution of our strategy and that the scientific team will continue its work on the determined drug research and discovery pipeline as planned. I would like to thank everyone for their support and look forward to being able to return to work as soon as possible.
Mr Johnny Cheng, Acting Chief Executive Officer and Chief Financial Officer, said, “Over the last 20 years under Dr Su’s leadership and the contribution of the entire team, HUTCHMED has built a portfolio of drugs and a strategy to successfully build new platforms and capabilities to deliver additional value. Together with our management team we shall endeavor to ensure on-track delivery. My best wishes to Dr Su for a speedy recovery.”
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This announcement contains forward-looking statements within the meaning of the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, the risk that current or future appointees to HUTCHMED’s board of directors are not effective in their respective positions, the difficulty in locating and recruiting suitable candidates for its board of directors and the management difficulties which may arise from changes in HUTCHMED’s board of directors. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the U.S. Securities and Exchange Commission, on AІM and with The Stock Exchange of Hong Kong Limited. HUTCHMED undertakes no obligation to update or revise the information contained in this announcement, whether as a result of new information, future events or circumstances or otherwise.
Inside Information
This announcement contains inside information for the purposes of Article 7 of Regulation (EU) No 596/2014 (as it forms part of retained EU law as defined in the European Union (Withdrawal) Act 2018).
Disclosure pursuant to the requirements of Rule 13.51(2) of the Rules Governing the Listing of Securities on The Stock Exchange of Hong Kong Limited
Mr Cheng, aged 58, has been an Executive Director since 2011 and Chief Financial Officer of the Company since 2008. He is a member of the Sustainability Committee of the Company. Prior to joining the Company, Mr Cheng was vice president, finance of Bristol Myers Squibb in China and was a director of Sino-American Shanghai Squibb Pharmaceuticals Ltd. and Bristol-Myers Squibb (China) Investment Co. Ltd. in Shanghai between late 2006 and 2008. Mr Cheng started his career as an auditor with Price Waterhouse (currently PricewaterhouseCoopers) in Australia and then KPMG in Beijing before spending eight years with Nestlé China where he was in charge of a number of finance and control functions in various operations. Mr Cheng received a Bachelor of Economics, Accounting Major from the University of Adelaide and is an associate of Chartered Accountants Australia and New Zealand. Mr Cheng does not have any relationship with any other Directors, senior management, substantial or controlling shareholders of the Company. As at the date of this announcement, Mr Cheng had a personal interest in 2,936,430 ordinary shares of the Company (“Shares”), representing approximately 0.34% of the issued Shares, within the meaning of Part XV of the Securities and Futures Ordinance (Chapter 571 of the Laws of Hong Kong). The term of Mr Cheng’s service as an Executive Director of the Company is subject to retirement by rotation and re-election at the annual general meeting of the Company in accordance with the provisions of the Articles of Association. The director’s fees of Mr Cheng as an Executive Director and a member of the Sustainability Committee of the Company under his appointment letter are US$70,000 and US$5,000 per annum respectively. The emoluments specified in the service agreement appointing Mr Cheng as Chief Financial Officer of the Company are US$490,513 per annum in salary and discretionary bonus which the Company may decide to pay. There will also be equity compensation of up to US$779,934 per annum. Such emoluments are determined by reference to the performance and profitability of the Company as well as his personal performance, remuneration benchmark in the industry and the prevailing market conditions. Such fees are subject to review from time to time and proration for an incomplete year of service. Save as disclosed above, there are no other matters concerning Mr Cheng that are required to be brought to the attention of the shareholders, nor is there other information that is required to be disclosed pursuant to the requirements of Rule 13.51(2) of the Rules Governing the Listing of Securities on The Stock Exchange of Hong Kong Limited.
Hong Kong, Shanghai & Florham Park, NJ — Wednesday, August 20, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces the completion of patient enrollment of SANOVO, a China Phase III study of ORPATHYS® (savolitinib) and TAGRISSO® (osimertinib) as a first-line treatment in certain non-small cell lung cancer (“NSCLC”) patients whose tumors harbor epidermal growth factor receptor (“EGFR”) mutation and MET overexpression. The last patient was enrolled on August 18, 2025.
This Phase III trial is a blinded, randomized, controlled study in previously untreated patients with locally advanced or metastatic NSCLC with activating EGFR mutations and MET overexpression. The study will evaluate the efficacy and safety of TAGRISSO® in combination with ORPATHYS® comparing to TAGRISSO® alone, a standard-of-care treatment option for these patients. The primary endpoint of the study is progression free survival (“PFS”) as assessed by investigators. Other endpoints include PFS assessed by an independent review committee, overall survival (OS), objective response rate (“ORR”), duration of response (DoR), disease control rate (DCR), time to response (TTR), and safety. Additional details may be found at clinicaltrials.gov, using identifier NCT05009836.
Topline results from the SANOVO study are estimated to be reported in the second half of 2026, followed by submission of results for presentation at an appropriate medical congress. If favorable, the results would enable a supplementary New Drug Application submission to China’s National Medical Products Administration (“NMPA”).
ORPATHYS® is an oral, potent and highly selective MET tyrosine kinase inhibitor (“TKI”) being jointly developed by AstraZeneca and HUTCHMED and commercialized by AstraZeneca. TAGRISSO® is a third-generation, irreversible EGFR TKI.
About NSCLC and MET aberrations
Lung cancer is the leading cause of cancer death, accounting for about one-fifth of all cancer deaths.[1] Lung cancer is broadly split into NSCLC and small cell lung cancer, with 80-85% classified as NSCLC.[2] The majority of NSCLC patients (approximately 75%) are diagnosed with advanced disease, and approximately 10-15% of NSCLC patients in the US and Europe and up to 40-50% of patients in Asia have EGFR-mutated (“EGFRm”) NSCLC.[3],[4],[5],[6],[7]
MET is a tyrosine kinase receptor that has an essential role in normal cell development. MET overexpression and/or amplification can lead to tumor growth and the metastatic progression of cancer cells, and is one of the mechanisms of de novo or acquired resistance to EGFR TKI for metastatic EGFRm NSCLC.[8],[9]
About ORPATHYS®
ORPATHYS® (savolitinib) is an oral, potent and highly selective MET TKI that has demonstrated clinical activity in advanced solid tumors. It blocks atypical activation of the MET receptor tyrosine kinase pathway that occurs because of mutations (such as exon 14 skipping alterations or other point mutations), gene amplification or protein overexpression.
ORPATHYS® is approved in China and is marketed by AstraZeneca for the treatment of adult patients with locally advanced or metastatic NSCLC with MET exon 14 skipping alteration, representing the first selective MET inhibitor approved in China. ORPATHYS® is also approved in China for the treatment of patients with locally advanced or metastatic EGFRm-positive non-squamous NSCLC with MET amplification after disease progression on EGFR tyrosine kinase inhibitor therapy, in combination with TAGRISSO®.
It is currently under clinical development for multiple tumor types, including lung, kidney, and gastric cancers as a single treatment and in combination with other medicines.
About TAGRISSO®
TAGRISSO® (osimertinib) is a third-generation, irreversible EGFR-TKI with proven clinical activity in NSCLC, including against central nervous system (CNS) metastases. TAGRISSO® (40mg and 80mg once-daily oral tablets) has been used to treat more than one million patients across its indications worldwide and AstraZeneca continues to explore TAGRISSO® as a treatment for patients across multiple stages of EGFRm NSCLC.
There is an extensive body of evidence supporting the use of TAGRISSO® in EGFRm NSCLC, and it is the only targeted therapy shown to improve patient outcomes across all stages of the disease.
In late-stage disease, TAGRISSO® demonstrated improved outcomes as monotherapy in the FLAURA Phase III trial and in combination with chemotherapy in the FLAURA2 Phase III trial. TAGRISSO® is also being investigated in this setting in combination with ORPATHYS® (savolitinib) in the SAFFRON Phase III trial and in combination with DATROWAY® (datopotamab deruxtecan or Dato-DXd) in the TROPION-Lung14 and TROPION-Lung15 Phase III trials.
TAGRISSO® also showed improved outcomes in early-stage disease in the NeoADAURA and ADAURA Phase III trials and in locally advanced stages in the LAURA Phase III trial. As part of AstraZeneca’s ongoing commitment to treating patients as early as possible in lung cancer, TAGRISSO® is also being investigated in the early-stage adjuvant resectable setting in the ADAURA2 Phase III trial.
About ORPATHYS® and TAGRISSO® Combination Development in EGFR-mutated NSCLC
Among patients who experience disease progression following treatment with a third-generation EGFR TKI, approximately 15-50% present with MET aberration, depending on the sample type, detection method and assay cut-off used. TAGRISSO® is a third-generation, irreversible EGFR-TKI with proven clinical activity in NSCLC, including against central nervous system metastases. Treatment with ORPATHYS® in combination with TAGRISSO® has been studied extensively in these patients in the TATTON (NCT02143466) and SAVANNAH (NCT03778229) studies. The encouraging results led to the initiation of several Phase III trials in this setting including the SACHI trial in China (NCT05015608) and the global SAFFRON trial (NCT05261399), as well as the SANOVO trial in China (NCT05009836).
This combination represents a promising chemotherapy-free oral treatment strategy to address mechanisms of resistance in this advanced setting. Positive data from the SACHI randomized Phase III trial led to the filing of a third NDA in China. Strong data from the SAVANNAH single-arm Phase II study was recently presented at the European Lung Cancer Congress (ELCC) in March 2025 demonstrated high, clinically meaningful and durable ORR, with consistent safety results. The SAFFRON randomized Phase III trial is progressing. Following AstraZeneca’s consultation with the US Food and Drug Administration (“FDA”), we look forward to completing the SAFFRON trial as soon as possible to support potential US and other global registration filings.
SACHI: The SACHI China Phase III study evaluated the combination of ORPATHYS® and TAGRISSO® for the treatment of patients with EGFRm, MET-amplified locally advanced or metastatic NSCLC after progression on EGFR TKI compared to platinum-based doublet chemotherapy. Results were presented at the ASCO Annual Meeting in June 2025. Based on the data from the SACHI study, the combination of ORPATHYS® and TAGRISSO® received approval from the China NMPA for the treatment of patients with locally advanced or metastatic EGFR mutation-positive non-squamous NSCLC with MET amplification after disease progression on EGFR TKI therapy in June 2025.
SAFFRON: In 2023, ORPATHYS® and TAGRISSO® received Fast Track Designation from the US FDA in this setting. The global SAFFRON Phase III trial is currently ongoing to assess the ORPATHYS® plus TAGRISSO® combination versus platinum-based doublet chemotherapy in patients with EGFRm, MET-overexpressed and/or amplified, locally advanced or metastatic NSCLC following progression on treatment with TAGRISSO®. Patients are being prospectively selected using the high MET level cut-off identified in SAVANNAH.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the therapeutic potential of ORPATHYS®, the further clinical development for ORPATHYS®, its expectations as to whether any studies on ORPATHYS® would meet their primary or secondary endpoints, and its expectations as to the timing of the completion and the release of results from such studies. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding enrollment rates and the timing and availability of subjects meeting a study’s inclusion and exclusion criteria; changes to clinical protocols or regulatory requirements; unexpected adverse events or safety issues; the ability of ORPATHYS®, including as a combination therapy, to meet the primary or secondary endpoint of a study, to obtain regulatory approval in other jurisdictions and to gain commercial acceptance after obtaining regulatory approval; the potential market of ORPATHYS® for a targeted indication; and HUTCHMED and/or its partner’s ability to fund, implement and complete its further clinical development and commercialization plans for ORPATHYS®, and the timing of these events. In addition, as certain studies rely on the use of other drug products such as TAGRISSO® as combination therapeutics with ORPATHYS®, such risks and uncertainties include assumptions regarding the safety, efficacy, supply and continued regulatory approval of these therapeutics. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
REFERENCES
[1] World Health Organization. International Agency for Research on Cancer. All cancers fact sheet. Available at: https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf. Accessed November 2022.
[2] American Cancer Society. What is Lung Cancer? Available at: https://www.cancer.org/cancer/lung-cancer/about/what-is.html. Accessed November 2022.
[3] Knight SB, et al. Progress and prospects of early detection in lung cancer. Open Biol. 2017;7(9): 170070.
[4] Keedy VL, et al. American Society of Clinical Oncology Provisional Clinical Opinion: Epidermal Growth Factor Receptor (EGFR) Mutation Testing for Patients with Advanced Non-Small-Cell Lung Cancer Considering First-Line EGFR Tyrosine Kinase Inhibitor Therapy. J Clin Oncol. 2011:29;2121-27.
[5] Zhang Y, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7(48).
[6] Szumera-Ciećkiewicz A, et al. EGFR Mutation Testing on Cytological and Histological Samples in 11. Non-Small Cell Lung Cancer: a Polish, Single Institution Study and Systematic Review of European Incidence. Int J Clin Exp Pathol. 2013:6;2800-12.
[7] Gou LY, et al. Prevalence of driver mutations in non-small-cell lung cancers in the People’s Republic of China. Lung Cancer: Targets and Therapy. 2014; 5; 1–9.
[8] Uchikawa E, et al. Structural basis of the activation of c-MET receptor. Nat Commun. 2021;12(4074).
[9] Wang Q, et al. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. Journal of Hematology & Oncology. 2019;63.
— Indications expansion driving growth and ATTC platform enriching pipeline —
— $455 million in net income attributable to HUTCHMED driven by non-core partial disposal —
Hong Kong, Shanghai & Florham Park, NJ — Thursday, August 7, 2025: HUTCHMED (China) Limited (“HUTCHMED”, the “Company” or “we”) (Nasdaq/AIM:HCM; HKEX:13) today reports its financial results for the six months ended June 30, 2025 and provides updates on key clinical and commercial developments.
HUTCHMED to host results webcasts today at 8:00 a.m. EDT / 1:00 p.m. BST / 8:00 p.m. HKT in English on Thursday, August 7, 2025, and tomorrow at 8:30 a.m. HKT in Chinese (Putonghua) on Friday, August 8, 2025. After registration, investors may access the live webcast at www.hutch-med.com/event.
All amounts are expressed in US dollars unless otherwise stated. A list of abbreviations is in the Glossary at the end of the page.
Global commercial progress and delivery of sustainable growth
- ORPATHYS® (savolitinib) secured China approval of its third lung cancer indication for EGFRm NSCLC patients with MET amplification after progression on EGFR inhibitor treatment in combination with TAGRISSO® (osimertinib) on June 30, 2025, in time to be eligible for potential national reimbursement negotiation towards the end of this year. This combination offers the only oral, chemotherapy-free approach to a sizable percentage (~30%) of these patients. The approval triggered a $11.0 million milestone payment from AstraZeneca which markets both ORPATHYS® and TAGRISSO®.
- FRUZAQLA® (fruquintinib ex-China) in-market sales by Takeda were up 25% to $162.8 million (H1-24: $130.5m) as its geographical coverage expanded to more than 30 countries. ELUNATE® (fruquintinib China) achieved $43.0 million (H1-24: $61.0m) reflecting intensifying competitive pressures and streamlining of our salesforce structure, but growth has returned recently. Total Oncology/Immunology consolidated revenue, including milestone and service income, was $143.5 million (H1-24: $168.7m).
- Net income attributable to HUTCHMED of $455.0 million was achieved in the first half of 2025 (H1-24: $25.8m), with a cash balance of $1.36 billion as of June 30, 2025, significantly boosted by a $416.3 million divestment gain, net of tax from the disposal of a partial equity stake in a non-core joint venture and divestment proceeds.
Pipeline progress and new technology platform
- Positive results from the SACHI China and SAVANNAH global lung cancer trials of ORPATHYS® in combination with TAGRISSO® were presented at ASCO and ELCC conferences. SACHI showed mPFS of 8.2 months with this oral combination compared to 3.0 months with chemotherapy, and SAVANNAH showed 7.4 months with this oral combination. This is the only treatment option that demonstrated statistically significant results in a biomarker-directed pivotal clinical trial in MET amplified, EGFR TKI refractory NSCLC patients. Enrollment in the SAFFRON global Phase III trial is expected to complete in the second half of this year and readout in the first half of 2026.
- Phase II/III trial on SULANDA® (surufatinib) in combination with AiRuiKa® (camrelizumab) and chemotherapy for previously-untreated metastatic pancreatic cancer patients is progressing well, targeting data readout in the second half of 2025. An earlier study presented promising updated data at ASCO with ORR of 51.1% (vs 24.4% with chemotherapy) and mPFS of 7.9 months (vs 5.4 months).
- Positive FRUSICA-2 Phase III results supported the China approval submission for ELUNATE® with TYVYT® (sintilimab) in previously-treated kidney cancer. Details to be presented at ESMO Congress. Prior Phase Ib/II study showed ORR of 60.0% and mPFS of 15.9 months.
- New Antibody-Targeted Therapy Conjugates (ATTC) platform drug candidates have been selected, planning to enter clinical development in late 2025. We also plan to present pre-clinical data at a scientific conference before the end of this year. Successful development of multiple ATTC molecules is expected to lead to collaboration and licensing opportunities in the future. Initial responses from potential partners are very positive.
Dr Dan Eldar, Non-executive Chairman of HUTCHMED, said, “With a strong balance sheet, robust operations and an exciting new ATTC platform, HUTCHMED is ready to enter a new phase of growth. Partnering is still a strategic focus, with multinational pharmaceutical companies remaining favorable towards such licensing opportunities with China biotech companies. In recent months we have seen markets’ sentiment and performance have significantly improved. China domestic drug policy and pricing environment also manifest strengthened support for innovative drug development, with the potential introduction of a commercial insurance drug list later this year, targeting a diversified, multi-layered healthcare social security payment system down the road.
We intend to prudently and actively deploy resources to expedite the development of a series of drug candidates from our novel ATTC platform, including synchronous clinical development in China and overseas. Our 20 years of knowledge of in-house discovery, experience in running large-scale pivotal trials, collaboration with international partners and success in obtaining global regulatory approvals empower us to bring forth more innovative medicines to address large unmet needs around the world.”
Dr Weiguo Su, Chief Executive Officer and Chief Scientific Officer of HUTCHMED, said, “We concluded the first half of 2025 with several important milestones achieved, some earlier than expected. The presentation of SACHI data at ASCO in a late-breaking oral presentation at the beginning of June was impressive, validating both the clinical strength and commercial advantages of ORPATHYS® in the market. This is the first biomarker-selected pivotal study globally for EGFR TKI refractory lung cancer patients, demonstrating clear clinical benefits for these patients. The China approval of ORPATHYS® at the end of June for this indication, six months after filing acceptance, was ahead of schedule and in time to qualify for national reimbursement negotiation. Also in June, the third indication of ELUNATE® for kidney cancer was accepted for review by the NMPA, supported by positive data in the FRUSICA-2 Phase III trial, to be presented at ESMO Congress. We also launched TAZVERIK® (tazemetostat), our first hematological oncology drug, in July following approval in March.
We believe sales growth should improve in second half of 2025, with the help of indication expansion in China and better market penetration overseas. In the near term, we shall start clinical development of multiple drug candidates from our ATTC program, a crucial technology platform, which will enrich our pipeline and provide ample partnership opportunities.”
2025 Interim Results & Business Updates
I. COMMERCIAL OPERATIONS
FRUZAQLA® in-market sales by Takeda were up 25% in the first half of 2025 at $162.8 million, driven by strong growth following approvals in more than 30 countries to date, including over 10 new markets in 2025. Reimbursement was received in the US, Spain and Japan last year, and, in July 2025, positive recommendation was received for NHS reimbursement in England and Wales.
The China pharmaceutical sector has gone through multifaceted changes. To position HUTCHMED for sustainable long-term growth, HUTCHMED has streamlined its sales force to establish a more efficient commercial organization and enhance productivity. In the face of intensifying competition as its products mature, HUTCHMED has strengthened its strategy to continue to focus on science-driven commercial activities to further differentiate its products. In the first half of 2025, in-market sales in China for ELUNATE®, SULANDA® and ORPATHYS® decreased as compared to the first half of 2024, reflecting competition and the transitional effects of the changes in our sales team and marketing strategy.
Total in-market sales were down 4%. Consolidated revenue dropped 22% due to lower China in-market sales, offset by flat FRUZAQLA® revenue.
Other Oncology/Immunology revenue, consisting of upfront or milestones, R&D services and licensing to our partners increased 9% to $44.4 million. Revenue from Other Ventures, comprising prescription drug distribution, remained flat, leading to total consolidated revenue of $277.7 million, down 9%.
* FRUZAQLA®, ELUNATE® and ORPATHYS® mainly represent total sales to third parties as provided by Takeda, Eli Lilly and AstraZeneca, respectively.
** FRUZAQLA® represents manufacturing revenue and royalties paid by Takeda; ELUNATE® represents manufacturing revenue, promotion and marketing services revenue and royalties paid by Eli Lilly to HUTCHMED, and sales to other third parties invoiced by HUTCHMED; ORPATHYS® represents manufacturing revenue and royalties paid by AstraZeneca to HUTCHMED and sales to other third parties invoiced by HUTCHMED; SULANDA® and TAZVERIK® represent the HUTCHMED’s sales of the products to third parties.
II. REGULATORY UPDATES
- Savolitinib sNDA approved by the NMPA for 2L EGFRm NSCLC patients with MET amplification, in combination with TAGRISSO®, triggering $11.0 million milestone from AstraZeneca, in June 2025.
- Savolitinib sNDA approved by the NMPA for 1L and 2L (converted from conditional to full approval) METex14 NSCLC in January 2025. Savolitinib approved in Hong Kong for METex14 NSCLC under the 1+ Mechanism in February 2025.
- Tazemetostat NDA conditionally approved by the NMPA for 3L R/R follicular lymphoma with EZH2 mutation in March 2025.
III. LATE-STAGE CLINICAL DEVELOPMENT ACTIVITIES
Savolitinib (ORPATHYS® in China), a highly selective oral inhibitor of MET
- Presented SACHI China Phase III results at ASCO 2025 for 2L EGFRm NSCLC patients with MET amplification, in combination with TAGRISSO®, showing mPFS of 8.2 months compared to 4.5 months with chemotherapy in ITT population (HR 0.34), and 6.9 months compared to 3.0 months in post third-generation EGFR TKI-treated subgroup (HR 0.32, both p<0.0001) (NCT05015608).
- Presented SAVANNAH global Phase II results at ELCC 2025 for 2L EGFRm NSCLC patients with MET amplification or overexpression, in combination with TAGRISSO®, showing ORR of 56%, mPFS of 7.4 months and mDoR of 7.1 months (NCT03778229).
- Continued enrolling SAFFRON global Phase III study for 2L EGFRm NSCLC patients with MET amplification or overexpression (NCT05261399) and the study will potentially support global filings; and SANOVO China Phase III study for 1L EGFRm NSCLC patients with MET overexpression (NCT05009836).
- Completed enrollment of China Phase II registrational study for 3L gastric cancer patients with MET amplification (NCT04923932).
Potential upcoming clinical milestones for savolitinib:
- Complete SAFFRON Phase III enrollment in the second half of 2025, data readout in the first half of 2026.
- Complete SANOVO China Phase III enrollment in the second half of 2025.
Fruquintinib (ELUNATE® in China, FRUZAQLA® outside of China), a selective oral inhibitor of VEGFR
- Positive results of FRUSICA-2 China Phase III in 2L RCC in March 2025 (NCT05522231).
- Presented China Phase II IIT results at AACR, in combination with TUOYI® (toripalimab) or TYVYT®, in 2L and above MSS/pMMR CRC, showing mPFS of 13.2 months and mOS of 29.0 months (NCT04483219).
Sovleplenib (HMPL-523), an investigative and highly selective oral inhibitor of Syk
- Ongoing ESLIM-01 ITP NMPA NDA review stipulates a lower impurity limit, requiring further manufacturing validation and stability test. Target re-submission in first half of 2026, with additional data rolling in during second half of 2026. In the future, the company will look to continue overseas development.
- Published China Phase II results in warm AIHA in China at EHA and in The Lancet Haematology in 2025, demonstrating overall response rate of 66.7% and a favorable safety profile (NCT05535933).
- Completed ESLIM-02 China Phase III enrollment for warm AIHA in June 2025 (NCT05535933).
Potential upcoming regulatory milestones for sovleplenib:
- ESLIM-01 NMPA NDA re-submission in first half of 2026 (NCT05029635).
- ESLIM-02 NMPA sNDA submission in first half of 2026 (NCT05535933).
Surufatinib (SULANDA® in China), an oral inhibitor of VEGFR, FGFR and CSF-1R
Potential upcoming clinical milestone for surufatinib:
- Data readout of Phase II part of a China Phase II/III HUTCHMED-sponsored trial for 1L metastatic PDAC patients, in combination with AiRuiKa®, nab-paclitaxel and gemcitabine in late 2025 (NCT06361888).
Tazemetostat (TAZVERIK® in China), a first-in-class, oral inhibitor of EZH2
- TAZVERIK® NDA approved by the NMPA for 3L R/R follicular lymphoma with EZH2 mutation.
- Continued enrolling SYMPHONY-1 China portion of the Phase III portion of the global study, in combination with lenalidomide and rituximab, in 2L follicular lymphoma patients (NCT04224493).
Fanregratinib (HMPL-453), a novel, highly selective and potent inhibitor targeting FGFR 1, 2 and 3
- Completed enrollment of China Phase II registrational trial for IHCC with FGFR fusion / rearrangement in February 2025 (NCT04353375).
Ranosidenib (HMPL-306), an investigative and highly selective oral dual-inhibitor of IDH1 and IDH2 enzymes
- Continued enrolling RAPHAEL China Phase III trial for 2L R/R IDH1/2-mutant AML (NCT06387069).
IV. ANTIBODY-TARGETED THERAPY CONJUGATE (ATTC) PLATFORM
New in-house created platform with multiple potential IND candidates
HUTCHMED plans to initiate China and global clinical trials for our first ATTC drug candidate around the end of 2025, followed by multiple global IND filings for more ATTC candidates in 2026.
Our ATTC next-generation technology platform leverages over 20 years of expertise in targeted therapies with small molecules inhibitors. By linking a monoclonal antibody with a proprietary targeted small-molecule inhibitor (SMI) payload, our ATTC platform has the capability to derive multiple drug candidates targeting various oncology indications, including precision medicine against selective sub-types. These ATTC drug candidates enrich the next wave of clinical development with potential key advantages over traditional antibody-drug conjugates and/or small molecule medicines.
- Better efficacy through synergistic antibody-small molecule targeted therapy combinations that will target specific mutations; overcome drug resistance to existing treatment.
- Improved safety and prolonged treatment given lower off-tumor or off-target toxicity than small molecules, lower risk of myelosuppression and better safety than cytotoxin-based conjugates.
- Attractive pharmacokinetics tackles difficult drug targets, enabled by antibody-guided delivery to target sites which will improve bioavailability and reduce drug-drug interactions.
- Advantages over existing ADCs due to lower off-tumor toxicities from the SMI payload, released through lysosomal cleavage inside target cells, targets cell signaling pathway driven by mutation specific to tumor cells. It can be used in combination with established standard therapies such as chemotherapy and immunotherapy to further enhance efficacy.
- Potential first-line applications, as chemo-free ATTC can potentially support combinations with other targeted therapies, chemotherapy and immunotherapy, in early-line settings with broad market potential.
V. COLLABORATION UPDATES
Further progress of candidate IMG-007, discovered by HUTCHMED
- ImageneBio, Inc. (Nasdaq: IMA) – Inmagene and Ikena Oncology, Inc. completed a merger on July 25, 2025 and ImageneBio, Inc., the merged entity, holds the license rights to IMG-007 granted by HUTCHMED. HUTCHMED has an approximate 3.67% shareholding in ImageneBio, Inc.
- Announced positive results of a US/Canada Phase IIa study of IMG-007 for atopic dermatitis in April 2025, showing week 16 mean change in EASI of 77% and EASI-75 response of 54% (NCT05984784).
- Dosed the first patient in a US Phase IIb randomized, double-blind, placebo-controlled dose-finding study of IMG-007 for moderate-to-severe atopic dermatitis in July 2025, targeting to enroll 220 patients who have had inadequate response to and/or intolerance of topical therapies (NCT07037901).
- Announced positive results of a US/Canada Phase IIa study of IMG-007 for severe alopecia areata in January 2025, showing mean reduction from baseline in Severity of Alopecia Tool (SALT) score of 30.1% by week 36 (NCT06060977).
VI. OTHER VENTURES
- Other Ventures consolidated revenue, predominantly from the prescription drug distribution business in China, were steady at $134.2 million for the six months ended June 30, 2025.
- HUTCHMED divested a 45.0% equity interest in SHPL for $608.5 million in cash in April 2025, retaining a 5.0% equity interest. A divestment gain, net of tax of $416.3 million was recognized during the first half of 2025. As a result, HUTCHMED’s share of equity in earnings of SHPL decreased to $23.1 million for the six months ended June 30, 2025.
- Consolidated net income attributable to HUTCHMED from Other Ventures increased to $440.3 million (H1-24: $34.1m), primarily due to the SHPL interest disposal.
VII. SUSTAINABILITY
In April 2025, the 2024 Sustainability Report was published, highlighting the progress made in 11 goals and targets and enhanced climate actions, including improved Scope 3 data, tightened control over air travel and engagement with suppliers. This year, a comprehensive climate risks assessment is being conducted to further understand and quantify the potential financial impacts of climate change, including physical risks brought by flooding and heat stress, and transition risks for HUTCHMED under optimistic and pessimistic scenarios.
HUTCHMED has made notable progress in its ESG ratings, including ratings from CDP Worldwide, the Hang Seng Corporate Sustainability Index Series, ISS ESG, MSCI ESG, Sustainalytics, and S&P Global ESG. In May 2025, HUTCHMED ranked third in ESG Excellence in the Healthcare, Pharmaceutical, and Biotechnology sector in the Extel’s Asia Executive Team survey, reflecting feedback from over 5,400 portfolio managers and analysts. Extel ranked HUTCHMED as one of the Most Honored Companies; ranked it first in Best Board of Directors, Best CEO, Best IR Program and Best IR Professionals; as well as second in Best CFO and Best IR Team in the Healthcare, Pharmaceutical, and Biotechnology sector.
Financial Highlights
Revenue for the six months ended June 30, 2025 was $277.7 million compared to $305.7 million for the six months ended June 30, 2024.
- Oncology/Immunology consolidated revenue amounted to $143.5 million (H1-24: $168.7m):
- FRUZAQLA® revenue was $43.1 million (H1-24: $42.8m), reflecting continued growth in royalties, offset by reduced manufacturing revenue compared to its launch year. In-market sales by Takeda were $162.8 million (up 25%) driven by strong growth following approvals in more than 30 countries to date, including over 10 new markets in 2025.
- ELUNATE® revenue decreased to $33.6 million (H1-24: $46.0m) in its seventh year since launch, comprising manufacturing revenue, promotion and marketing services revenue and royalties. In-market sales decreased to $43.0 million, reflecting the intensifying competitive pressures from combination therapies of key competing products and their additional generics and biosimilars entry in 3L CRC. The launch of the entry of the new indication 2L EMC in 2025 and continuous inclusion of ELUNATE® in key guidelines are expected to drive future growth.
- SULANDA® revenue decreased to $12.7 million (H1-24: $25.4m) in the face of strong competition for NET patients from new somatostatin analogues drugs with their inclusion in the NRDL and broader coverage. To counteract this challenge, we continue to drive awareness and product differentiation to uphold SULANDA® position in TKI.
- ORPATHYS® revenue decreased to $9.0 million (H1-24: $13.1m) on in-market sales of $15.2 million, impacted by the launch and NRDL inclusion of several competing drugs for METex14 skipping Such results have not reflected expected growth from the recent approval for the much larger EGFR TKI-refractory, MET-amplified NSCLC patient population at the end of June 2025.
- TAZVERIK® revenue was $0.7 million (H1-24: $0.5m) mainly from sales in Hainan and Hong Kong. Launched in mainland China in July 2025 following its approval in March 2025.
- Takeda upfront, regulatory milestones and R&D services revenue were $29.5 million (H1-24: $33.8m), of which $26.6 million was recognized from Takeda deferred revenue.
- Other revenue of $14.9 million (H1-24: $7.1m), includes regulatory milestone of $11.0 million from AstraZeneca following China NDA approval for ORPATHYS® combined with TAGRISSO®.
- Other Ventures consolidated revenue of $134.2 million (H1-24: $137.0m) remained flat.
Net Expenses for the six months ended June 30, 2025 were $239.0 million compared to $279.9 million for the six months ended June 30, 2024, reflecting strong cost control efforts.
- Cost of Revenue decreased 7% to $167.6 million (H1-24: $180.1m), which was mainly due to lower Oncology/Immunology revenue. Cost of revenue as a percentage of oncology product revenue remained stable at 39% (H1-24: 38%).
- R&D Expenses reduced by 24% to $72.0 million (H1-24: $95.3m). While R&D investment outside of China reduced to $7.6 million (H1-24: $14.9m) as we continued to integrate our global R&D operations with China, the decrease was mainly driven by China with R&D investment of $64.4 million (H1-24: $80.4m) reflecting lower costs from completed studies which are under NDA review (e.g. ELUNATE® in 2L RCC) or already led to NMPA approval in H1-25 (e.g. ORPATHYS® in 2L NSCLC). Joint China and global clinical development effort ongoing to gear up for multiple drug candidates from our ATTC
- S&A Expenses were $41.6 million (H1-24: $57.8m). The decrease was mainly due to a reduction in S&A expenses for oncology products which was $13.4 million or 13.5% of oncology product revenue (H1-24: $25.1 million or 19.6%) as sales force structure was streamlined and tighter spending controls imposed.
- Other Items generated net income of $42.2 million (H1-24: $53.3m), mainly comprised of equity in earnings of SHPL, interest income and expense, foreign exchange and taxes. The decrease was primarily due to lower share of equity in earnings of SHPL at $23.1 million (H1-24: $33.8m) as our share decreased to 5% (H1-24: 50%) after the divestment of a partial stake in SHPL completed in April 2025.
Gain on divestment of SHPL, net of tax was $416.3 million for the six months ended June 30, 2025.
Net Income attributable to HUTCHMED for the six months ended June 30, 2025 was $455.0 million compared to $25.8 million for the six months ended June 30, 2024.
- The net income attributable to HUTCHMED for the six months ended June 30, 2025 was $0.53 per ordinary share / $2.65 per ADS (H1-24: $0.03 per ordinary share / $0.15 per ADS).
Cash, Cash Equivalents and Short-Term Investments were $1,364.5 million as of June 30, 2025 compared to $836.1 million as of December 31, 2024.
- Adjusted Group (non-GAAP) net cash inflows excluding financing activities in the first half of 2025 were $519.1 million mainly due to the receipt of $608.5 million gross proceeds from the partial divestment of SHPL, offset with the $59.5 million capital gain tax payment for the partial divestment of SHPL, $10.0 million regulatory approval milestone payment and $9.2 million in capital expenditures (H1-24: -$51.3m mainly due to $39.8 million net cash used in operating activities and $10.1 million of capital expenditures).
- Net cash generated from financing activities in the first half of 2025 totaled $9.3 million mainly due to drawdowns of bank borrowings of $8.2 million (H1-24: net cash used in financing activities of $32.6m mainly due to purchases for equity awards of $36.1 million).
Foreign exchange impact: The RMB depreciated against the US dollar on average by approximately 0.8% during the first half of 2025, which has impacted consolidated financial results as highlighted.
FINANCIAL GUIDANCE
HUTCHMED updates full year 2025 guidance for Oncology/Immunology consolidated revenue to $270 million – $350 million due to the phasing of milestone income from partners to 2026 and onwards, as well as the estimated delay of sovleplenib China NDA review completion to after 2025. HUTCHMED will leverage its strong cash resources to accelerate ATTC global development and explore investment opportunities.
Shareholders and investors should note that:
- The Company does not provide any guarantee that the statements contained in the financial guidance will materialize or that the financial results contained therein will be achieved or are likely to be achieved; and
- The Company has in the past revised its financial guidance and reference should be made to any announcements published by it regarding any updates to the financial guidance after the date of publication of this announcement.
———
Use of Non-GAAP Financial Measures and Reconciliation – References in this announcement to adjusted Group net cash flows excluding financing activities and financial measures reported at CER are based on non-GAAP financial measures. Please see the “Use of Non-GAAP Financial Measures and Reconciliation” for further information relevant to the interpretation of these financial measures and reconciliations of these financial measures to the most comparable GAAP measures, respectively.
———
Financial Summary
Condensed Consolidated Balance Sheets Data
Condensed Consolidated Statements of Operations Data
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, and the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
References
Unless the context requires otherwise, references in this announcement to the “Group,” the “Company,” “HUTCHMED,” “HUTCHMED Group,” “we,” “us,” and “our,” mean HUTCHMED (China) Limited and its subsidiaries unless otherwise stated or indicated by context.
Past Performance and Forward-Looking Statements
The performance and results of operations of the Group contained within this announcement are historical in nature, and past performance is no guarantee of future results of the Group. This announcement contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements can be identified by words like “will,” “expects,” “anticipates,” “future,” “intends,” “plans,” “believes,” “estimates,” “pipeline,” “could,” “potential,” “first-in-class,” “best-in-class,” “designed to,” “objective,” “guidance,” “pursue,” or similar terms, or by express or implied discussions regarding potential drug candidates, potential indications for drug candidates or by discussions of strategy, plans, expectations or intentions. You should not place undue reliance on these statements. Such forward-looking statements are based on the current beliefs and expectations of management regarding future events, and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. There can be no guarantee that any of our drug candidates will be approved for sale in any market, that any approvals which have been obtained will continue to remain valid and effective in the future, or that the sales of products marketed or otherwise commercialized by HUTCHMED and/or its collaboration partners (collectively, “HUTCHMED’s Products”) will achieve any particular revenue or net income levels. In particular, management’s expectations could be affected by, among other things: unexpected regulatory actions or delays or government regulation generally; the uncertainties inherent in research and development, including the inability to meet our key study assumptions regarding enrollment rates, timing and availability of subjects meeting a study’s inclusion and exclusion criteria and funding requirements, changes to clinical protocols, unexpected adverse events or safety, quality or manufacturing issues; the delay or inability of a drug candidate to meet the primary or secondary endpoint of a study; the delay or inability of a drug candidate to obtain regulatory approval in different jurisdictions or the utilization, market acceptance and commercial success of HUTCHMED’s Products after obtaining regulatory approval; discovery, development and/or commercialization of competing products and drug candidates that may be superior to, or more cost effective than, HUTCHMED’s Products and drug candidates; the impact of studies (whether conducted by HUTCHMED or others and whether mandated or voluntary) or recommendations and guidelines from governmental authorities and other third parties on the commercial success of HUTCHMED’s Products and drug candidates in development; the ability of HUTCHMED to manufacture and manage supply chains, including various third party services, for multiple products and drug candidates; the availability and extent of reimbursement of HUTCHMED’s Products from third-party payers, including private payer healthcare and insurance programs and government insurance programs; the costs of developing, producing and selling HUTCHMED’s Products; the ability to obtain additional funding when needed; the ability to obtain and maintain protection of intellectual property for HUTCHMED’s Products and drug candidates; the ability of HUTCHMED to meet any of its financial projections or guidance and changes to the assumptions underlying those projections or guidance; the successful disposition of its non-core business; global trends toward health care cost containment, including ongoing pricing pressures; uncertainties regarding actual or potential legal proceedings, including, among others, actual or potential product liability litigation, litigation and investigations regarding sales and marketing practices, intellectual property disputes, and government investigations generally; and general economic and industry conditions, including uncertainties regarding the effects of the persistently weak economic and financial environment in many countries, uncertainties regarding future global exchange rates, uncertainties in global interest rates, and geopolitical relations, sanctions and tariffs. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, on AIM and on HKEX. HUTCHMED is providing the information in this announcement as of this date and does not undertake any obligation to update any forward-looking statements as a result of new information, future events or otherwise.
In addition, this announcement contains statistical data and estimates that HUTCHMED obtained from industry publications and reports generated by third-party market research firms. Although HUTCHMED believes that the publications, reports and surveys are reliable, HUTCHMED has not independently verified the data and cannot guarantee the accuracy or completeness of such data. You are cautioned not to give undue weight to this data. Such data involves risks and uncertainties and are subject to change based on various factors, including those discussed above.
Inside Information
This announcement contains inside information for the purposes of Article 7 of Regulation (EU) No 596/2014 (as it forms part of retained EU law as defined in the European Union (Withdrawal) Act 2018).
Medical Information
This announcement contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
🔻GLOSSARY (click to unfold)🔻
1L = First-line.
2L = Second-line.
3L = Third-line.
AACR = American Association for Cancer Research.
ADC = Antibody-drug conjugate.
ADS = American depositary shares, each of which represents five ordinary shares.
AIHA = Autoimmune hemolytic anemia.
AKT = Protein kinase B.
AML = Acute myeloid leukemia.
ASCO = American Society of Clinical Oncology.
ASH = American Society of Hematology.
AstraZeneca = AstraZeneca AB, a subsidiary of AstraZeneca plc.
ATTC = Antibody-targeted therapy conjugates.
BICR = Blinded independent central review.
BOC = Bank of China Limited.
BTK = Bruton’s tyrosine kinase.
CDP Worldwide = formerly known as the Carbon Disclosure Project.
CER = Constant exchange rate. We also report changes in performance at CER which is a non-GAAP measure. Please refer to “Use of Non-GAAP Financial Measures and Reconciliation” for further information relevant to the interpretation of these financial measures and reconciliations of these financial measures to the most comparable GAAP measures.
CLL = Chronic lymphocytic leukemia.
CNS = Central nervous system.
CR+CRh = Combined complete remission + complete remission with partial hematologic recovery.
CRC = Colorectal cancer.
CSF-1R = Colony-stimulating factor 1 receptor.
DCR = Disease control rate.
DLBCL = Diffuse large B-cell lymphoma.
DoR = Duration of response.
EASI = Eczema area and severity index.
EGFR = Epidermal growth factor receptor.
EGFRm = Epidermal growth factor receptor mutated.
EHA = European Hematology Association.
ELCC = The European Lung Cancer Congress.
Eli Lilly = Lilly (Shanghai) Management Company Limited.
EMC = Endometrial cancer.
epNET = Extra-pancreatic neuroendocrine tumor.
Epizyme = Epizyme, Inc., an Ipsen company.
ERK = Extracellular signal-regulated kinase.
ESG = Environmental, Social and Governance.
ESMO = European Society for Medical Oncology.
EZH2 = Enhancer of zeste homolog 2.
EZH2m = Enhancer of zeste homolog 2 mutated.
FPI = First patient in.
FDA = Food and Drug Administration.
FGFR = Fibroblast growth factor receptor.
FLT3 = FMS-like tyrosine kinase 3.
GAAP = Generally Accepted Accounting Principles.
GC = Gastric cancer.
HR = Hazard Ratio.
Hainan Pilot Zone = Hainan Boao Lecheng International Medical Tourism Pilot Zone.
HKEX = The Main Board of The Stock Exchange of Hong Kong Limited.
ICML = International Conference on Malignant Lymphoma.
IDH1/2 = Isocitrate dehydrogenase-1 OR isocitrate dehydrogenase-2.
IHCC = Intrahepatic cholangiocarcinoma.
IIT = Investigator-initiated trial.
IND = Investigational new drug application.
Inmagene = Inmagene Biopharmaceuticals.
In-market sales = Total sales to third parties provided by Eli Lilly (ELUNATE®), Takeda (FRUZAQLA®), AstraZeneca (ORPATHYS®) and HUTCHMED (ELUNATE®, SULANDA®, ORPATHYS® and TAZVERIK®).
Ipsen = Ipsen SA, parent of Epizyme, Inc.
IRC = Independent review committee.
ITP = Immune thrombocytopenia purpura.
ITT = Intend-to-treat.
JAK = Janus kinase.
JAMA = Journal of the American Medical Association.
LPI = Last patient in.
LPR = Loan Prime Rate.
MAPK = Mitogen-activated protein kinase.
mDoR = median Duration of response.
MET = Mesenchymal epithelial transition factor.
METex14 = MET exon 14 skipping alteration.
MLL = Mixed-lineage leukemia.
mOS = median Overall survival.
mPFS = median Progression-free survival.
MSS = Microsatellite stable.
NDA = New Drug Application.
NET = Neuroendocrine tumor.
NHL = Non-Hodgkin lymphoma.
NHS = National Health Service in the United Kingdom.
NHSA = China National Healthcare Security Administration.
NMPA = China National Medical Products Administration.
NPM1 = Nucleophosmin 1.
NRDL = China National Reimbursement Drug List.
NSCLC = Non-small cell lung cancer.
ORR = Objective response rate.
OS = Overall survival.
PBOC = People’s Bank of China.
PD-1 = Programmed cell death protein-1.
PDAC = Pancreatic ductal adenocarcinoma.
PFS = Progression free survival.
PI3K = Phosphatidylinositol 3-kinase.
PI3Kδ = Phosphoinositide 3-kinase-δ.
pMMR = Proficient mismatch repair.
pNET = Pancreatic neuroendocrine tumor.
PRCC = Papillary renal cell carcinoma.
PTCL = Peripheral T-cell lymphomas.
R/R = Relapsed and/or refractory.
RAS = Rat sarcoma.
RCC = Renal cell carcinoma.
RMB or “renminbi” = The legal currency of the PRC.
RP2D = The recommended phase 2 dose.
RP3D = The recommended phase 3 dose.
S&A = Selling and administrative expenses.
SHP2 = SH2 containing protein tyrosine phosphatase-2.
SHPL = Shanghai Hutchison Pharmaceuticals Limited.
SLL = Small lymphocytic lymphoma.
sNDA = Supplemental New Drug Application.
STAT = Signal transducer and activator of transcription.
Syk = Spleen tyrosine kinase.
Takeda = Takeda Pharmaceuticals International AG, a subsidiary of Takeda Pharmaceutical Company Limited.
TEAE = Treatment emergent adverse events.
TKI = Tyrosine kinase inhibitor.
TPO = Thrombopoietin.
TPO-RA = Thrombopoietin receptor agonists.
TRAE = Treatment-related adverse events.
VEGFR = Vascular endothelial growth factor receptor.
wAIHA = Warm autoimmune haemolytic anaemia.
WCLC = World Conference on Lung Cancer.
| Announcement release: August 7, 2025 (Thu) |
| 7am EDT / 12 noon BST / 7pm HKT |
| View Announcement |
| Presentation webcast & call |
| ⯈ English Session: August 7, 2025 (Thu) |
| 8am EDT / 1pm BST / 8pm HKT |
| ⯈ Chinese (Putonghua) Session: August 8, 2025 (Fri) |
| 8:30am HKT / 1:30am BST / (8:30pm EDT on Aug 7) |
Hong Kong, Shanghai & Florham Park, NJ — Thursday, July 3, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM: HCM; HKEX:13) will be announcing its interim results for the six months ended June 30, 2025 on Thursday, August 7, 2025 at 7:00 am Eastern Daylight Time (EDT) / 12:00 noon British Summer Time (BST) / 7:00 pm Hong Kong Time (HKT).
Management team of HUTCHMED will host a presentation for analysts and investors to discuss the interim financial results, followed by a Q&A session.
The English webcast will be held on Thursday, August 7, 2025, at 8:00 am EDT (1:00 pm BST / 8:00 pm HKT). The Chinese (Putonghua) webcast will be held at 8:30 am HKT / 1:30 am BST on Friday, August 8, 2025 (8:30 pm EDT on Thursday, August 7, 2025). Both webcasts will be available live via the company website at www.hutch-med.com/event/. The presentation will be available for downloading before the webcast begins. Details of the webcast will be provided in the financial results announcement and on the company website. A replay will also be available on the website shortly after the event.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch-med.com or follow us on LinkedIn.
CONTACTS
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
HSBC |
Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
| Cavendish | Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
| Deutsche Numis | Joint Broker |
| Freddie Barnfield / Jeffrey Wong / Duncan Monteith | +44 20 7260 1000 |
Hong Kong, Shanghai & Florham Park, NJ — Wednesday, July 2, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that it has appointed Deutsche Bank AG (trading as Deutsche Numis) as its joint Corporate Broker in London with immediate effect following the termination of HSBC Bank plc as joint Corporate Broker. Panmure Liberum Limited and Cavendish Capital Markets Limited will continue to act as joint Corporate Brokers in London and Panmure Liberum Limited will continue to act as Nominated Advisor to HUTCHMED in London in respect of the AIM rules.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery, global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception, HUTCHMED has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit www.hutch-med.com or follow us on LinkedIn.
CONTACTS
| Investor Enquiries | +852 2121 8200 / ir@hutch-med.com |
| Media Enquiries | |
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
| Panmure Liberum | Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
| HSBC | Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
| Cavendish | Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
| Deutsche Numis | Joint Broker |
| Freddie Barnfield / Jeffrey Wong / Duncan Monteith | +44 20 7260 1000 |
— Approval based on Phase III SACHI Trial results which showed a 66% reduced risk of progression or death as compared to platinum-based chemotherapy —
— The only all-oral combination treatment option for these patients —
— Consistent benefit regardless of first-line EGFR inhibitor therapy —
Hong Kong, Shanghai & Florham Park, NJ — Monday, June 30, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that the New Drug Application (“NDA”) for the combination of ORPATHYS® (savolitinib) and TAGRISSO® (osimertinib) has been granted approval by the China National Medical Products Administration (“NMPA”) for the treatment of patients with locally advanced or metastatic epidermal growth factor receptor (“EGFR”) mutation-positive non-squamous non-small cell lung cancer (“NSCLC”) with MET amplification after disease progression on EGFR tyrosine kinase inhibitor (“TKI”) therapy. ORPATHYS® is an oral, potent and highly selective MET TKI. TAGRISSO® is a third-generation, irreversible EGFR TKI. This approval also triggers a US$11 million milestone payment from AstraZeneca, which markets both ORPATHYS® and TAGRISSO® in China.
ORPATHYS® was the first selective MET inhibitor approved in China, indicated for adult patients with locally advanced or metastatic NSCLC with MET exon 14 skipping alteration. This new approval by the NMPA was based on data from the SACHI Phase III trial of the ORPATHYS® and TAGRISSO® combination (NCT05015608), having met the pre-defined primary endpoint of progression-free survival (“PFS”) in a pre-planned interim analysis. Primary results were presented at the American Society of Clinical Oncology (“ASCO”) Annual Meeting in June 2025. In 2024 the NMPA designated the combination as a Breakthrough Therapy, and in 2025 it granted the NDA Priority Review.
Professor Shun Lu, Chief of the Shanghai Lung Cancer Center at Shanghai Chest Hospital, School of Medicine, Shanghai Jiaotong University, and Principal Investigator of the SACHI trial, said, “The approval of the ORPATHYS® and TAGRISSO® combination is a significant milestone in addressing the complex challenges of lung cancer treatment in China, where the EGFR mutation is common amongst NSCLC patients. For patients who develop MET amplification after progressing on EGFR inhibitors, the combination offers a continued all-oral, chemotherapy-free approach to tackle a critical resistance mechanism. As a researcher and clinician, I am excited about the opportunity to offer this targeted therapy to patients, improving their treatment outcomes and quality of life through innovative research.”
“The NMPA approval marks an important step forward in our mission to address MET-driven progression following first-line EGFR-inhibitor therapy in NSCLC patients.” said Dr Weiguo Su, Chief Executive Officer and Chief Scientific Officer of HUTCHMED. “Our collaboration with AstraZeneca, built on a shared vision to transform oncology care, has been crucial in reaching this achievement. We are committed to advancing this partnership, continuing our research into further treatment settings, and bringing this innovative combination to patients in China and beyond.”
Ms Mary Guan, General Manager of AstraZeneca China Oncology Business, said: “This milestone marks the third indication of ORPATHYS® approved in China, bringing a new treatment option to lung cancer patients who develop MET amplification after progressing on EGFR inhibitor therapy. Through our partnership with HUTCHMED, we are committed to expanding the reach of the ORPATHYS® and TAGRISSO® combination to address progression on first-line therapy and help even more patients with this form of lung cancer.”
In the intention to treat (ITT) population of the SACHI trial, the ORPATHYS® and TAGRISSO® combination reduced the risk of disease progression by 66% with a median PFS of 8.2 months, compared to 4.5 months for chemotherapy, as assessed by investigators. The independent review committee (IRC) also reported a 60% risk reduction in disease progression, with a median PFS of 7.2 months versus 4.2 months, respectively. The safety profile of the ORPATHYS® and TAGRISSO® combination was tolerable and no new safety signals were observed. Treatment-emergent adverse events of Grade 3 or above occurred in 57% of patients in both the ORPATHYS® plus TAGRISSO® group and the chemotherapy group, suggesting a favorable safety profile.
About NSCLC and MET aberrations
Lung cancer is the leading cause of cancer death, accounting for about one-fifth of all cancer deaths.[1] Lung cancer is broadly split into NSCLC and small cell lung cancer, with 80-85% classified as NSCLC.[2] The majority of NSCLC patients (approximately 75%) are diagnosed with advanced disease, and approximately 10-15% of NSCLC patients in the US and Europe and 30-40% of patients in Asia have EGFR-mutated (“EGFRm”) NSCLC.[3],[4],[5],[6]
MET is a tyrosine kinase receptor that has an essential role in normal cell development. MET overexpression and/or amplification can lead to tumor growth and the metastatic progression of cancer cells, and is one of the mechanisms of acquired resistance to EGFR TKI for metastatic EGFRm NSCLC.[7],[8]
About ORPATHYS®
ORPATHYS® (savolitinib) is an oral, potent and highly selective MET TKI that has demonstrated clinical activity in advanced solid tumors. It blocks atypical activation of the MET receptor tyrosine kinase pathway that occurs because of mutations (such as exon 14 skipping alterations or other point mutations), gene amplification or protein overexpression.
ORPATHYS® is approved in China and is marketed by AstraZeneca for the treatment of adult patients with locally advanced or metastatic NSCLC with MET exon 14 skipping alteration, representing the first selective MET inhibitor approved in China. It is currently under clinical development for multiple tumor types, including lung, kidney, and gastric cancers as a single treatment and in combination with other medicines.
About TAGRISSO®
TAGRISSO® (osimertinib) is a third-generation, irreversible EGFR-TKI with proven clinical activity in NSCLC, including against central nervous system (CNS) metastases. TAGRISSO® (40mg and 80mg once-daily oral tablets) has been used to treat nearly 800,000 patients across its indications worldwide and AstraZeneca continues to explore TAGRISSO® as a treatment for patients across multiple stages of EGFRm NSCLC.
There is an extensive body of evidence supporting the use of TAGRISSO® as standard of care in EGFRm NSCLC. TAGRISSO® improved patient outcomes in early-stage disease in the ADAURA Phase III trial, locally advanced disease in the LAURA Phase III trial, late-stage disease in the FLAURA Phase III trial, and with chemotherapy in the FLAURA2 Phase III trial.
About ORPATHYS® and TAGRISSO® Combination Development in EGFR-mutated NSCLC
Among patients who experience disease progression following treatment with a third-generation EGFR TKI, approximately 15-50% present with MET aberration, depending on the sample type, detection method and assay cut-off used. TAGRISSO® is a third-generation, irreversible EGFR-TKI with proven clinical activity in NSCLC, including against central nervous system metastases. Treatment with ORPATHYS® in combination with TAGRISSO® has been studied extensively in these patients in the TATTON (NCT02143466) and SAVANNAH (NCT03778229) studies. The encouraging results led to the initiation of several Phase III trials in this setting including the SACHI trial in China (NCT05015608) and the global SAFFRON trial (NCT05261399), as well as the SANOVO trial in China (NCT05009836).
This combination represents a promising chemotherapy-free oral treatment strategy to address mechanisms of resistance in this advanced setting. Positive data from the SACHI randomized Phase III trial led to the filing of a second NDA in China. Strong data from the SAVANNAH single-arm Phase II study was recently presented at the European Lung Cancer Congress (ELCC) in March 2025 demonstrated high, clinically meaningful and durable objective response rate (ORR), with consistent safety results. The SAFFRON randomized Phase III trial is progressing. Following AstraZeneca’s consultation with the US Food and Drug Administration (“FDA”), we look forward to completing the SAFFRON trial as soon as possible to support potential US and other global registration filings.
SACHI: The SACHI China Phase III trial met the primary endpoint of PFS during its interim analysis towards the end of 2024 and a NDA was accepted and granted Breakthrough Therapy Designation and Priority Review status in China in December 2024. SACHI evaluated the combination of ORPATHYS® and TAGRISSO® for the treatment of patients with EGFRm, MET-amplified locally advanced or metastatic NSCLC after progression on EGFR TKI compared to platinum-based doublet chemotherapy. Results were presented at the ASCO Annual Meeting in June 2025.
SAFFRON: In 2023, ORPATHYS® and TAGRISSO® received Fast Track Designation from the US FDA in this setting. The global SAFFRON Phase III trial is currently ongoing to assess the ORPATHYS® plus TAGRISSO® combination versus platinum-based doublet chemotherapy in patients with EGFRm, MET-overexpressed and/or amplified, locally advanced or metastatic NSCLC following progression on treatment with TAGRISSO®. Patients are being prospectively selected using the high MET level cut-off identified in SAVANNAH.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This announcement contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the therapeutic potential of ORPATHYS®, the further clinical development for ORPATHYS®, its expectations as to whether any studies on ORPATHYS® would meet their primary or secondary endpoints, and its expectations as to the timing of the completion and the release of results from such studies. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding enrollment rates and the timing and availability of subjects meeting a study’s inclusion and exclusion criteria; changes to clinical protocols or regulatory requirements; unexpected adverse events or safety issues; the ability of ORPATHYS®, including as a combination therapy, to meet the primary or secondary endpoint of a study, to obtain regulatory approval in other jurisdictions and to gain commercial acceptance after obtaining regulatory approval; the potential market of ORPATHYS® for a targeted indication; and HUTCHMED and/or its partner’s ability to fund, implement and complete its further clinical development and commercialization plans for ORPATHYS®, and the timing of these events. In addition, as certain studies rely on the use of other drug products such as TAGRISSO® as combination therapeutics with ORPATHYS®, such risks and uncertainties include assumptions regarding the safety, efficacy, supply and continued regulatory approval of these therapeutics. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this announcement, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This announcement contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
Inside Information
This announcement contains inside information for the purposes of Article 7 of Regulation (EU) No 596/2014 (as it forms part of retained EU law as defined in the European Union (Withdrawal) Act 2018).
CONTACTS
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Emma Earl / Rupert Dearden | +44 20 7886 2500 |
Cavendish |
Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
REFERENCE
[1] World Health Organization. International Agency for Research on Cancer. All cancers fact sheet. Available at: https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf. Accessed November 2022.
[2] American Cancer Society. What is Lung Cancer? Available at: https://www.cancer.org/cancer/lung-cancer/about/what-is.html. Accessed November 2022.
[3] Knight SB, et al. Progress and prospects of early detection in lung cancer. Open Biol. 2017;7(9): 170070.
[4] Keedy VL, et al. American Society of Clinical Oncology Provisional Clinical Opinion: Epidermal Growth Factor Receptor (EGFR) Mutation Testing for Patients with Advanced Non-Small-Cell Lung Cancer Considering First-Line EGFR Tyrosine Kinase Inhibitor Therapy. J Clin Oncol. 2011:29;2121-27.
[5] Zhang Y, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7(48).
[6] Szumera-Ciećkiewicz A, et al. EGFR Mutation Testing on Cytological and Histological Samples in 11. Non-Small Cell Lung Cancer: a Polish, Single Institution Study and Systematic Review of European Incidence. Int J Clin Exp Pathol. 2013:6;2800-12.
[7] Uchikawa E, et al. Structural basis of the activation of c-MET receptor. Nat Commun. 2021;12(4074).
[8] Wang Q, et al. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. Journal of Hematology & Oncology. 2019;63.
Hong Kong, Shanghai, & Florham Park, NJ: Tuesday, June 10, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM: HCM; HKEX: 13) announces that on June 9, 2025, it granted share options (“Share Options”) under the Share Option Scheme adopted by HUTCHMED in 2015 (the “Share Option Scheme”) and awards (“LTIP Awards”) under the Long Term Incentive Plan adopted by HUTCHMED in 2025 (“LTIP”).
Aimed at attracting and retaining top talent, the Remuneration Committee of HUTCHMED appointed an independent advisor to conduct compensation benchmarking research on a selected peer group of companies. As a result of this the Remuneration Committee has comprehensively reviewed the compensation and share-based incentives policies of HUTCHMED and its subsidiaries (the “Group”) and established an attractive policy to ensure the Group is able to recruit and retain top talent. In line with this review HUTCHMED has decided to make the following grant of Share Options and LTIP Awards.
1. Performance Related Share Options
HUTCHMED granted Share Options subject to the Performance Targets (defined below) under its Share Option Scheme to Dr Weiguo Su (Executive Director, Chief Executive Officer and Chief Scientific Officer), being a person discharging managerial responsibility (“PDMR”) under the UK Market Abuse Regulation to subscribe for 1,493,435 ordinary shares with par value US$0.10 each in the share capital of the Company (“Ordinary Shares”) subject to the acceptance of the grantee. Details of such Share Options granted are as follows:
| Date of grant | : | June 9, 2025 |
| Exercise price of share options granted | : | HK$25.50 per Ordinary Share |
| Number of share options granted | : | 1,493,435 Ordinary Shares |
| Closing market price of Ordinary Shares at HKEX on the date of grant | : | HK$25.50 per Ordinary Share |
| Exercise period of the share options | : | From June 9, 2025 to June 8, 2035 |
| Vesting period of the share options | : | Vesting will occur two business days after the date of announcement of the annual results of the Company for the financial year ending December 31, 2027 (the “2027 Results Announcement”). |
| Performance targets | : | The exercise of the share options is conditional upon the fulfilment of certain performance targets relating to the Group over the financial years 2025 to 2027 (the “Performance Targets”). The number of share options to be exercisable will be determined on the date of 2027 Results Announcement. The Performance Targets have been determined by the Board and specified in the grant letter of Dr Su. To the extent that the Performance Targets have not been met, the relevant number of share options granted to Dr Su will lapse. |
| Clawback mechanism | : | The share options may be subject to the clawback policy of the Company enabling the Company to recover incentive-based compensation paid to covered executive officers in the event of mis-statement of the financial statements of the Company resulting from material non-compliance with financial reporting requirements. |
After the above grant of Share Options, the number of Share Options available for future grant under the scheme mandate of the Share Option Scheme is 11,409,825.
2. Non-performance-related LTIP Award (“Performance LTIP Award”) – award based on a maximum cash amount, which amount is determined by the achievement of performance targets for the financial years ending December 31, 2025, 2026 and 2027. The performance targets are determined by the Remuneration Committee of HUTCHMED based on the strategic objectives of HUTCHMED.
The shares, to be purchased by the trustee following determination of the cash amount based on actual achievement of performance targets, will then be held by the trustee until the related underlying LTIP Awards are vested. Vesting will occur three weeks after the date of completion of the share purchase for the awards for the financial year ending December 31, 2027. Vesting will also depend upon the continued employment of the award holder with the Group and will otherwise be at the discretion of the Board of Directors of HUTCHMED.
HUTCHMED granted the following Performance LTIP Awards to the following Executive Directors, each being a PDMR under the UK Market Abuse Regulation:
| Award Holder | Maximum amount for the Performance LTIP Awards | |
| Dr Weiguo Su (Executive Director, Chief Executive Officer and Chief Scientific Officer) | US$3,442,787 | |
| Mr Johnny Cheng (Executive Director and Chief Financial Officer) | US$779,934 |
An additional 132 employees of the Group have simultaneously been granted Performance LTIP Awards.
The notification in respect of share options granted to Dr Su in accordance with the requirements of the UK Market Abuse Regulation is set out below.
Dr Weiguo Su
| 1 | Details of the person discharging managerial responsibilities/person closely associated | |||||
| a) | Name | Dr Weiguo Su | ||||
| 2 | Reason for the notification | |||||
| a) | Position/status | Executive Director, Chief Executive Officer and Chief Scientific Officer | ||||
| b) | Initial notification/Amendment | Initial notification | ||||
| 3 | Details of the issuer, emission allowance market participant, auction platform, auctioneer or auction monitor | |||||
| a) | Name | HUTCHMED (China) Limited | ||||
| b) | LEI | 2138006X34YDQ6OBYE79 | ||||
| 4 | Details of the transaction(s): section to be repeated for (i) each type of instrument; (ii) each type of transaction; (iii) each date; and (iv) each place where transactions have been conducted | |||||
| a) |
Description of the financial instrument, type of instrument Identification code |
Share option over Ordinary Shares of US$0.10
Share option over Ordinary Share with DI ISIN: KYG4672N1198 |
||||
| b) | Nature of the transaction |
Grant of options in respect of 1,493,435 Ordinary Shares under the Share Option Scheme. The exercise of the share options is conditional upon the fulfilment of certain performance targets relating to the Group over the financial years 2025 to 2027 (the “Performance Targets”). The number of share options to be exercisable will be determined on the date of 2027 Results Announcement. The Performance Targets have been determined by the Board and specified in the grant letter of Dr Su. To the extent that the Performance Targets have not been met, the relevant number of share options granted to Dr Su will lapse. |
||||
| c) | Price(s) and volume(s) |
|
||||
| d) | Aggregated information
|
N/A | ||||
| e) | Date of the transaction | 2025-06-09 | ||||
| f) | Place of the transaction | Outside a trading venue | ||||
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This announcement contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. Forward-looking statements involve risks and uncertainties. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, on AIM and on HKEX. HUTCHMED undertakes no obligation to update or revise the information contained in this announcement, whether as a result of new information, future events or circumstances or otherwise.
CONTACTS
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Emma Earl / Rupert Dearden | +44 20 7886 2500 |
HSBC |
Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
Cavendish |
Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
Hong Kong, Shanghai & Florham Park, NJ — Thursday, June 5, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) and Innovent Biologics, Inc. (“Innovent”) (HKEX: 01801) today jointly announce that the New Drug Application (“NDA”) for the combination of fruquintinib and sintilimab for the treatment of patients with locally advanced or metastatic renal cell carcinoma who have failed prior treatment with one tyrosine kinase inhibitor (“TKI”) has been accepted for review by the China National Medical Products Administration (“NMPA”).
The NDA is supported by data from FRUSICA-2, a randomized, open-label, active-controlled registration study evaluating the efficacy and safety of fruquintinib in combination with sintilimab versus axitinib or everolimus monotherapy for the second-line treatment of advanced renal cell carcinoma. The study has met its primary endpoint of progression free survival (“PFS”), as assessed by blinded independent central review (BICR) according to RECIST 1.1 criteria. The combination also demonstrated improvements in secondary endpoints including objective response rate (“ORR”) and duration of response (“DoR”). The safety profile was tolerable and no new safety signals were observed. Data from FRUSICA-2 will be submitted for presentation at an upcoming scientific conference. Additional details may be found at clinicaltrials.gov, using identifier NCT05522231.
“Kidney cancer continues to pose significant challenges in China, with limited treatment options for patients who fail first-line therapies. Submitting this NDA for the fruquintinib and sintilimab combination for advanced renal cell carcinoma marks an important step in our efforts to address this unmet need,” said Dr Michael Shi, Head of R&D and Chief Medical Officer of HUTCHMED. “We are dedicated to making this combination therapy available to patients with renal cell carcinoma. At the same time, through ongoing research, we remain focused on exploring the full potential of this combination, as well as advancing our broader pipeline across multiple cancer types, to provide more patients with new and effective treatment options.”
“The NDA acceptance of sintilimab and fruquintinib combination represents a significant step toward providing a more effective second line treatment option for patients with advanced renal cell carcinoma in China,” said Dr Hui Zhou, Senior Vice President of Innovent. “Our PD-1 inhibitor, sintilimab (TYVYT®), has solidified its position as a cornerstone of immuno-oncology (IO) therapy with this NDA as its 10th indication, marking a meaningful milestone in lifecycle management and clinical value optimization.”
In December 2024, the combination of fruquintinib and sintilimab received conditional approval from the China NMPA for the treatment of patients with advanced mismatch repair proficient (“pMMR”) endometrial cancer who have failed prior systemic therapy and are not candidates for curative surgery or radiation, based on data from the FRUSICA-1 study (NCT03903705).
About Kidney Cancer and Renal Cell Carcinoma
It is estimated that approximately 435,000 new patients were diagnosed with kidney cancer worldwide in 2022.[1] In China, an estimated 74,000 new patients were diagnosed with kidney cancer in 2022.[2] Approximately 90% of kidney tumors are renal cell carcinoma.
About Fruquintinib
Fruquintinib is a selective oral inhibitor of all three vascular endothelial growth factor receptors (“VEGFR”) -1, -2 and -3. VEGFR inhibitors play a pivotal role in inhibiting tumor angiogenesis. Fruquintinib was designed to have enhanced selectivity that limits off-target kinase activity, allowing for drug exposure that achieves sustained target inhibition and flexibility for potential use as part of a combination therapy.[3]
About Fruquintinib Approvals
Fruquintinib is co-developed and co-commercialized in China by HUTCHMED and Eli Lilly and Company under the brand name ELUNATE®. It is approved for the treatment of patients with metastatic colorectal cancer who have previously received fluoropyrimidine, oxaliplatin and irinotecan-based chemotherapy, and those who have previously received or are not suitable to receive anti-VEGF therapy or anti-epidermal growth factor receptor (EGFR) therapy (RAS wild-type) in China. It was included in the China National Reimbursement Drug List (NRDL) in January 2020. Since its launch in China, over 100,000 patients with colorectal cancer have been treated with fruquintinib.
The combination of ELUNATE® (fruquintinib) and TYVYT® (sintilimab injection) has conditional approval in China for the treatment of patients with advanced pMMR endometrial cancer who have failed prior systemic therapy and are not candidates for curative surgery or radiation.
Takeda holds the exclusive worldwide license to further develop, commercialize, and manufacture fruquintinib outside mainland China, Hong Kong and Macau, marketing it under the brand name FRUZAQLA®. Fruquintinib received approval for the treatment of previously treated metastatic colorectal cancer in the US, Europe, Japan and many other countries around the world.
The safety and efficacy of fruquintinib for the following investigational uses have not been established and there is no guarantee that it will receive health authority approval or become commercially available in any country for the uses being investigated:
About Fruquintinib for Second-line Treatment of Renal Cell Carcinoma
Single-agent targeted therapy continues to be one of the primary choices for first-line treatment of advanced renal cell carcinoma in China. Notably, advanced renal cell carcinoma patients who have experienced failure with single-agent targeted therapy previously still indicate an unmet medical need.
Results from a proof-of-concept Phase Ib/II study of fruquintinib plus sintilimab were published in Targeted Oncology in January 2025. The combination showed promising efficacy and a tolerable safety profile in this setting. At the data cutoff of October 9, 2024, all 20 enrolled previously treated patients were evaluable for efficacy, with a median follow-up duration of 45.7 months. The confirmed ORR was 60.0% and DCR was 85.0%. Median DoR was 13.9 months and median PFS was 15.9 months. Overall survival (“OS”) was not reached, and the 36-month OS rate was 58.3%.[4]
About Sintilimab
Sintilimab, marketed as TYVYT® (sintilimab injection) in China, is a PD-1 immunoglobulin G4 monoclonal antibody co-developed and co-commercialized by Innovent and Eli Lilly and Company, which binds to PD-1 molecules on the surface of T-cells, blocks the PD-1 / PD-Ligand 1 (PD-L1) pathway, and reactivates T-cells to kill cancer cells. [5]
In China, sintilimab has been approved and included in the updated NRDL for seven indications. The updated NRDL reimbursement scope for TYVYT® (sintilimab injection) includes:
- For the treatment of relapsed or refractory classic Hodgkin’s lymphoma after two lines or later of systemic chemotherapy;
- For the first-line treatment of unresectable locally advanced or metastatic non-squamous non-small cell lung cancer lacking EGFR or ALK driver gene mutations;
- For the treatment of patients with EGFR-mutated locally advanced or metastatic non-squamous non-small cell lung cancer who progressed after EGFR-TKI therapy;
- For the first-line treatment of unresectable locally advanced or metastatic squamous non-small cell lung cancer;
- For the first-line treatment of unresectable or metastatic hepatocellular carcinoma with no prior systematic treatment;
- For the first-line treatment of unresectable locally advanced, recurrent or metastatic esophageal squamous cell carcinoma;
- For the first-line treatment of unresectable locally advanced, recurrent or metastatic gastric or gastroesophageal junction adenocarcinoma.
Furthermore, sintilimab’s eighth indication, in combination with fruquintinib for the treatment of patients with advanced endometrial cancer with pMMR tumors that have failed prior systemic therapy and are not candidates for curative surgery or radiation, was conditional approved by the NMPA in December 2024.
Two NDAs for sintilimab are currently under the NMPA review, including:
- In combination with ipilimumab as neoadjuvant treatment for resectable MSI-H/dMMR colon cancer is under the NMPA review and has been granted Priority Review designation;
- In combination with fruquintinib for the treatment of patients with locally advanced or metastatic renal cell carcinoma who failed prior treatment with a TKI.
In addition, two clinical studies of sintilimab have met their primary endpoints:
- Phase 2 study of sintilimab monotherapy as second-line treatment of esophageal squamous cell carcinoma;
- Phase 3 study of sintilimab monotherapy as second-line treatment for squamous non-small cell lung cancer with disease progression following platinum-based chemotherapy;
Statement: Innovent does not recommend the use of any unapproved drug(s)/indication(s).
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery, global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception, HUTCHMED has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit www.hutch-med.com or follow us on LinkedIn.
About Innovent
Innovent is a leading biopharmaceutical company founded in 2011 with the mission to empower patients worldwide with affordable, high-quality biopharmaceuticals. The company discovers, develops, manufactures and commercializes innovative medicines that target some of the most intractable diseases. Its pioneering therapies treat cancer, cardiovascular and metabolic, autoimmune and eye diseases. Innovent has launched 15 products in the market. It has 3 new drug applications under regulatory review, 4 assets in Phase III or pivotal clinical trials and 15 more molecules in early clinical stage. Innovent partners with over 30 global healthcare companies, including Lilly, Sanofi, Incyte, LG Chem and MD Anderson Cancer Center.
Guided by the motto, “Start with Integrity, Succeed through Action,” Innovent maintains the highest standard of industry practices and works collaboratively to advance the biopharmaceutical industry so that first-rate pharmaceutical drugs can become widely accessible. For more information, visit www.innoventbio.com, or follow Innovent on Facebook and LinkedIn.
Statement:
(1) Innovent does not recommend the use of any unapproved drug (s)/indication(s).
(2) Ramucirumab (Cyramza®) and Selpercatinib (Retsevmo®) and Pirtobrutinib (Jaypirca®) were developed by Eli Lilly and Company.
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the therapeutic potential of the fruquintinib and sintilimab combination for the treatment of patients with advanced renal cell carcinoma and the further clinical development of the fruquintinib and sintilimab combination in this and other indications. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding the sufficiency of clinical data to support NDA approval of the fruquintinib and sintilimab combination for the treatment of patients with advanced renal cell carcinoma in China, or other jurisdictions, its potential to gain expeditious approvals from regulatory authorities, the safety profile of fruquintinib, HUTCHMED’s ability to fund, implement and complete its further clinical development and commercialization plans for the fruquintinib and sintilimab combination, and the timing of these events. In addition, as certain studies rely on the use of other drug products such as sintilimab as combination therapeutics with fruquintinib, such risks and uncertainties include assumptions regarding the safety, efficacy, supply and continued regulatory approval of these therapeutics. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the U.S. Securities and Exchange Commission, on AIM and on The Stock Exchange of Hong Kong Limited. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
CONTACTS
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
HSBC |
Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
Cavendish |
Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
[1] The Global Cancer Observatory, kidney cancer fact sheet. https://gco.iarc.who.int/media/globocan/factsheets/cancers/29-kidney-fact-sheet.pdf. Accessed February 19, 2025.
[2] The Global Cancer Observatory, China fact sheet. https://gco.iarc.who.int/media/globocan/factsheets/populations/160-china-fact-sheet.pdf. Accessed February 19, 2025.
[3] Sun Q, et al. Discovery of fruquintinib, a potent and highly selective small molecule inhibitor of VEGFR 1, 2, 3 tyrosine kinases for cancer therapy. Cancer Biol Ther. 2014;15(12):1635-45. doi: 10.4161/15384047.2014.964087.
[4] Xu H, et al. Fruquintinib Plus Sintilimab in Patients with Treatment‑Naïve and Previously Treated Advanced Renal Cell Carcinoma: Results from a Phase Ib/II Clinical Trial. Targeted Oncology. 2025; 20:113–125. doi.org/10.1007/s11523-024-01120-6.
[5] Wang J, et al. Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. mAbs 2019;11(8): 1443-1451. doi: 10.1080/19420862.2019.1654303.
| Date: Tuesday, June 3, 2025 |
| Time: 8:30-9:00 am HKT |
| (8:30-9:00 pm EDT on Monday, June 2, 2025) |
— The all-oral chemotherapy-free combination of savolitinib plus osimertinib demonstrated significant PFS benefit with a favorable safety profile in the SACHI Phase III China study —
— Webcast to be held at 8:30 am HKT on Tuesday, June 3 to discuss the data presented —
Hong Kong, Shanghai & Florham Park, NJ — Monday, June 2, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) announces primary results from the interim analysis of the SACHI Phase III study. These results were presented in a late-breaking oral presentation on Sunday, June 1, 2025, during the American Society of Clinical Oncology (“ASCO”) Annual Meeting in Chicago, USA.
SACHI is a Phase III study of the savolitinib and osimertinib combination for the treatment of patients with locally advanced or metastatic epidermal growth factor receptor (“EGFR”) mutation-positive non-small cell lung cancer (“NSCLC”) with MET amplification after disease progression on first-line EGFR inhibitor therapy (clinicaltrials.gov identifier NCT05015608).
| Title: | Savolitinib combined with osimertinib versus chemotherapy in EGFR-mutant and MET-amplification advanced NSCLC after disease progression on EGFR tyrosine kinase inhibitor: Results from a randomized Phase III SACHI study |
| Lead Author: | Shun Lu, Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China |
| Session: | Oral Abstract Session: Lung Cancer – Non-Small Cell Metastatic |
| Abstract Number: | LBA8505 |
| Date & Time: | Sunday, June 1, 2025, 8:00 AM Central Daylight Time |
| Location: | Arie Crown Theater |
Prof. Shun Lu, Chief of the Shanghai Lung Cancer Center at Shanghai Chest Hospital, School of Medicine, Shanghai Jiaotong University, and Principal Investigator of the SACHI study, said, “The results from the SACHI Phase III study represent a significant advancement in the treatment of EGFR mutation-positive NSCLC with MET amplification. The savolitinib and osimertinib combination demonstrates promising efficacy in patients who have progressed on prior EGFR inhibitor therapy. These findings highlight the potential of this novel, chemotherapy-free combination to enable a continued oral regimen, offering a convenient and well-tolerated treatment option that addresses critical unmet needs for patients with this challenging disease.”
HUTCHMED will host a webcast to discuss the data presented at the ASCO Annual Meeting at 8:30 -9:00 am HKT on Tuesday, June 3, 2025 (8:30 – 9:00 pm EDT on June 2, 2025). The event will be held in English and can be accessed via www.hutch-med.com/event. A replay will also be available on the website shortly after the event.
As of the interim analysis data cut-off of August 30, 2024, a total of 211 patients were randomized to receive the savolitinib and osimertinib combination or chemotherapy. In the intention to treat (ITT) population, the median progression-free survival (“PFS”) assessed by investigator was 8.2 months with savolitinib plus osimertinib, compared to 4.5 months with chemotherapy (hazard ratio [“HR”] 0.34; 95% confidence interval [“CI”] 0.23-0.49; p < 0.0001). The independent review committee (“IRC”) assessed median PFS was 7.2 months vs 4.2 months, respectively (HR 0.40; 95% CI 0.28-0.59; p < 0.0001).
The investigator-assessed objective response rate (ORR) was 58% in the savolitinib plus osimertinib group compared to 34% for patients in the chemotherapy group. The disease control rate (DCR) was 89% vs 67% and the median duration of response (DoR) was 8.4 months vs 3.2 months, respectively. Overall survival was not mature at the time of the interim analysis.
Efficacy outcomes in the third-generation EGFR tyrosine kinase inhibitor (“TKI”)–treated patients were comparable with those in the intention-to-treat and third-generation EGFR-TKI–naïve populations. In the third generation EGFR-TKI–treated subgroup, the investigator-assessed and IRC-assessed median PFS were highly consistent, both at 6.9 vs 3.0 months (HR 0.32; p < 0.0001).
The safety profile of the savolitinib and osimertinib combination was tolerable and no new safety signals were observed. Treatment-emergent adverse events of Grade 3 or above occurred in 57% of patients in the savolitinib plus osimertinib group compared to 57% for patients in the chemotherapy group, suggesting a favorable safety profile.
In January 2025, the Independent Data Monitoring Committee (IDMC) of SACHI has considered that the study has met the pre-defined primary endpoint of PFS in a planned interim analysis and as a result, enrollment into the study has concluded. Supported by data from SACHI, a New Drug Application (NDA) for the combination of savolitinib and osimertinib for the treatment of patients with locally advanced or metastatic EGFR mutation-positive NSCLC with MET amplification after disease progression on first-line EGFR inhibitor therapy has been accepted and granted priority review by the China National Medical Products Administration (NMPA).
About Savolitinib
Savolitinib is an oral, potent, and highly selective MET TKI that has demonstrated clinical activity in advanced solid tumors. MET is a tyrosine kinase receptor that has an essential role in normal cell development. Savolitinib blocks atypical activation of the MET receptor tyrosine kinase pathway that occurs because of mutations (such as exon 14 skipping alterations or other point mutations), gene amplification or protein overexpression. MET overexpression and/or amplification can lead to tumor growth and the metastatic progression of cancer cells, and is a known mechanism of acquired resistance to EGFR TKIs. The prevalence of MET depends on the sample type, detection method and assay cut-off used.
Savolitinib is approved in China and is marketed under the brand name ORPATHYS® by our partner, AstraZeneca, for the treatment of adult patients with locally advanced or metastatic NSCLC with MET exon 14 skipping alteration, representing the first selective MET inhibitor approved in China. It has been included in the National Reimbursement Drug List of China (NRDL) since March 2023.
It is currently under clinical development for multiple tumor types, including lung, kidney, and gastric cancers as a single treatment and in combination with other medicines.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including but not limited to its expectations regarding the therapeutic potential of savolitinib, the further clinical development for savolitinib, its expectations as to whether any studies on savolitinib would meet their primary or secondary endpoints, and its expectations as to the timing of the completion and the release of results from such studies. Such risks and uncertainties include, among other things, assumptions regarding enrollment rates and the timing and availability of subjects meeting a study’s inclusion and exclusion criteria; changes to clinical protocols or regulatory requirements; unexpected adverse events or safety issues; the ability of savolitinib, including as combination therapies, to meet the primary or secondary endpoint of a study, to obtain regulatory approval in different jurisdictions and to gain commercial acceptance after obtaining regulatory approval; the potential markets of savolitinib for a targeted indication, and the sufficiency of funding. In addition, as certain studies rely on the use of other drug products such as osimertinib as combination therapeutics, such risks and uncertainties include assumptions regarding their safety, efficacy, supply and continued regulatory approval. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
CONTACTS
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
HSBC |
Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
| Cavendish | Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
Hong Kong, Shanghai & Florham Park, NJ — Friday, May 30, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) hereby notifies the market that as at May 30, 2025, the issued share capital of HUTCHMED consisted of 871,611,095 ordinary shares of US$0.10 each, with each share carrying one right to vote and with no shares held in treasury.
The above figure of 871,611,095 may be used by shareholders as the denominator for the calculations by which they could determine if they are required to notify their interest in, or a change to their interest in, HUTCHMED shares under the Financial Conduct Authority’s Disclosure Guidance and Transparency Rules.
For illustrative purposes only, the 871,611,095 ordinary shares would be equivalent to 871,611,095 depositary interests (each equating to one ordinary share) which are traded on AIM or, if the depositary interests were converted in their entirety, equivalent to 174,322,219 American depositary shares (each equating to five ordinary shares) which are traded on Nasdaq.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Hong Kong, Shanghai & Florham Park, NJ — Friday, May 23, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that new data from several studies of compounds discovered by HUTCHMED including savolitinib, ranosidenib, fruquintinib and surufatinib, will be presented at the American Society of Clinical Oncology (“ASCO”) Annual Meeting taking place on May 30 – June 3, 2025 in Chicago, USA.
Results from the SACHI China Phase III study of savolitinib in combination with osimertinib in patients with locally advanced or metastatic epidermal growth factor receptor (“EGFR”) mutation-positive non-small cell lung cancer (“NSCLC”) with MET amplification after disease progression on EGFR inhibitor therapy will be presented at a late breaking oral presentation. SACHI had met the pre-defined primary endpoint of progression-free survival (PFS) in a planned interim analysis. SACHI data supports the New Drug Application (NDA) for this oral-only treatment, which has been accepted and granted priority review in China.
Further data with additional analysis stratified by brain metastasis status from a high MET overexpression and/or amplification treatment subset of the SAVANNAH Phase II study of the savolitinib and osimertinib combination in NSCLC patients harboring EGFR mutation and MET amplification or overexpression after progressing on osimertinib were reported. The savolitinib and osimertinib combination demonstrated better efficacy outcomes compared to savolitinib plus placebo. The combination showed promising central nervous system (“CNS”) activity, with reduced CNS progression and fewer new CNS lesions.
Results will be presented from the dose-escalation stage of the Phase I study of ranosidenib (HMPL-306), a novel, small-molecule, highly selective oral dual-inhibitor of both Isocitrate dehydrogenase (“IDH”) 1 and IDH2 enzymes, being studied in patients with locally advanced or metastatic solid tumors with IDH mutations. Results show that the compound was well tolerated, showing target inhibition and durable responses in patients. Efficacy signals were observed especially in the efficacy evaluated group of lower-grade glioma patients (N=14), with an objective response rate (“ORR”) of 7.1% and a disease control rate (“DCR”) of 100%.
Results will also be presented from the sub-group analyses of the FRUSICA-1 open-label, single-arm, pivotal Phase II study to evaluate the efficacy and safety of fruquintinib plus sintilimab in previously treated advanced endometrial cancer (EMC) patients with pMMR (proficient mismatch repair) status. Efficacy findings for patients with serous carcinoma (N=27) were clinically meaningful and characterized by responses similar to those observed in full trial population (N=98), with an Independent Review Committee (“IRC”)-assessed ORR of 37.0% and a DCR of 88.9%. The analysis of whether the response was affected by prior neoadjuvant/adjuvant chemotherapy (“NACT/ACT”) showed durable and clinically meaningful responses regardless of whether the patient had received NACT/ACT. Results were comparable for patients with and without prior NACT/ACT, with an IRC-assessed ORR of 34.0% versus 31.4% and DCR of 85.1% versus 82.4%, respectively.
Results from two subgroup analyses of a Phase IV study of fruquintinib involving 2,798 colorectal cancer patients in China will be presented. In the subgroup analysis evaluating the safety of fruquintinib as monotherapy and in combination therapy, fruquintinib demonstrated a manageable safety profile in both groups. Treatment-emergent adverse events (TEAE) of Grade 3 or above occurred in 23.94% in the fruquintinib monotherapy group and 26.06% in the combination therapy group with other anti-cancer treatments. The most common treatment related adverse events (TRAE) of any grade in both groups were palmar-plantar erythrodysesthesia (PPES) and hypertension. The combination therapy group exhibited a longer treatment duration, potentially indicating improved patient outcomes. In the subgroup analysis by age, the safety of fruquintinib was assessed in younger (age <50) and late-elderly (age ≥75) patients. The safety profile was comparable across both age groups, with younger patients receiving more intensive treatment. Combination therapy with fruquintinib also showed a longer duration than monotherapy in both age subgroups, which may suggest improved survival potential.
Details of the presentations, including links to available abstracts, are as follows:
| Abstract title | Presenter / Lead author | Presentation details |
| SPONSORED STUDIES | ||
| Savolitinib (Savo) combined with osimertinib (osi) versus chemotherapy (chemo) in EGFR-mutant (EGFRm) and MET-amplification (METamp) advanced NSCLC after disease progression (PD) on EGFR tyrosine kinase inhibitor (TKI): Results from a randomized phase 3 SACHI study | Shun Lu, Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China | LBA8505 Oral Abstract Session: Lung Cancer – Non-Small Cell Metastatic Sunday, June 1, 2025 9:48 AM CDT |
| Efficacy and CNS results from a randomized subset of the phase 2 SAVANNAH study comparing savolitinib (savo) + osimertinib (osi) combination with savo + placebo (PBO) | Benjamin Philip Levy, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore, MD | 8513 Rapid Oral Session: Lung Cancer – Non-Small Cell Metastatic Monday, June 2, 2025 8:06 AM CDT |
| Phase I study of HMPL-306, an inhibitor of mutant IDH1/IDH2 (mIDH1/2), in western patients (pts) with advanced mIDH solid tumor, including glioma | Jordi Rodon Ahnert, The University of Texas MD Anderson Cancer Center, Houston, TX | 2013 Rapid Oral Session: Central Nervous System Tumors Saturday, May 31, 2025 3:06 PM CDT |
| Analysis of serous carcinoma subgroup in FRUSICA-1: Fruquintinib plus sintilimab in treated advanced endometrial cancer (EMC) patients (pts) with pMMR status | Xiaohua Wu, Fudan University Shanghai Cancer Center, Shanghai, China | 5596 Poster Session: Gynecologic Cancer |
| The Impact of Prior Neoadjuvant/Adjuvant Chemotherapy (NACT/ACT) on Fruquintinib Plus Sintilimab Outcomes in Advanced Endometrial Cancer (EMC) Patients with pMMR Status: A Subgroup Analysis of FRUSICA-1 | Jing Wang, Hunan Cancer Hospital, Changsha, China | 5611 Poster Session: Gynecologic Cancer |
| Safety of fruquintinib in young and late-elderly Chinese patients with colorectal cancer in real-world clinical practice: Age subgroup analysis of a fruquintinib Phase IV study | Yi Wang, Ningbo No.2 Hospital, Ningbo, China | e15512 Publication Only: Gastrointestinal Cancer – Colorectal and Anal |
| Safety of fruquintinib monotherapy and combination therapy in Chinese Patients with colorectal cancer in real-world clinical practice: A subgroup analysis from Phase IV study | Zhiqiang Wang, Sun Yat-Sen University Cancer Center, Guangzhou, China | e15515 Publication Only: Gastrointestinal Cancer – Colorectal and Anal |
| The appropriate therapeutic sequence with angiogenesis inhibitor and chemotherapy in patients with advanced gastric or gastroesophageal junction adenocarcinoma: Exploratory analysis from the Phase III FRUTIGA study | Jin Li, Shanghai East Hospital, Tongji University, Shanghai, China | e16011 Publication Only: Gastrointestinal Cancer – Gastroesophageal, Pancreatic, and Hepatobiliary |
| Subgroup analysis of efficacy and safety of fruquintinib plus paclitaxel versus paclitaxel in gastroesophageal junction adenocarcinoma patients from FRUTIGA: A randomized Phase III clinical trial in second-line treatment of gastric/gastroesophageal junction | Tianshu Liu, Zhongshan Hospital, Fudan University, Shanghai, Shanghai, China | e16012 Publication Only: Gastrointestinal Cancer – Gastroesophageal, Pancreatic, and Hepatobiliary |
| INVESTIGATOR-INITIATED STUDIES | ||
| Fruquintinib in combination with camrelizumab and paclitaxel liposome and nedaplatin as first-line treatment for advanced esophageal squamous cell carcinoma (ESCC): a single-arm, Phase II study | Yanhong Gu, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China | 4042 Poster Session: Gastrointestinal Cancer – Gastroesophageal, Pancreatic, and Hepatobiliary |
| Updated results of fruquintinib combined with PD-1 inhibitors and chemotherapy in the first-line treatment of HER2-negative advanced gastric or gastroesophageal junction adenocarcinoma (FDZL-FIX): a single-arm, open-label Phase II study | Chenchen Wang, Fudan University Shanghai Cancer Center, Shanghai, China | 4046 Poster Session: Gastrointestinal Cancer – Gastroesophageal, Pancreatic, and Hepatobiliary |
| Open-label, single-arm, single-center Phase Ib/II clinical study of fruquintinib combined with trastuzumab and XELOX in the first-line treatment of advanced HER2-positive metastatic gastric or gastroesophageal junction adenocarcinoma | Huifang Lv, The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, China | TPS4203 Poster Session: Gastrointestinal Cancer – Gastroesophageal, Pancreatic, and Hepatobiliary |
| A multi-cohort real-world study of treatment for metastatic colorectal cancer (mCRC): Overall efficacy analysis and subgroup analysis of previous bevacizumab use or not | Wangxia Lv, The Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Hangzhou, China | e15530 Publication Only: Gastrointestinal Cancer – Colorectal and Anal |
| Real-world Observational Study of Fruquintinib in Combination with Irinotecan and Capecitabine as Second-line Treatment in Patients with Advanced Colorectal Cancer | Ling Xu, the First Hospital of China Medical University, Shenyang, China | e15539 Publication Only: Gastrointestinal Cancer – Colorectal and Anal |
| Preliminary results of fruquintinib in combination with FOLFIRI as second-line treatment for RAS-mutant metastatic colorectal cancer: a prospective single-center Phase II study | Ru Jia, Fifth Medical Center, Chinese PLA General Hospital, Beijing, China | e15541 Publication Only: Gastrointestinal Cancer – Colorectal and Anal |
| Evaluating the efficacy of fruquintinib versus regorafenib and trifluridine/tipiracil in treating advanced metastatic colorectal cancer: A match-adjusted indirect comparison | Shukui Qin, Gastrointestinal Cancer Center of Nanjing Tianyinshan Hospital, China Pharmaceutical University, Nanjing, China | e15550 Publication Only: Gastrointestinal Cancer – Colorectal and Anal |
| Fruquintinib plus sintilimab and SOX as conversion therapy for initially unresectable gastric/gastroesophageal junction adenocarcinoma (GC/GEJC): Updated response and surgical results from a single-arm, Phase II clinical trial | Fei Ma, Henan Cancer Hospital, Zhengzhou, China | e16016 Publication Only: Gastrointestinal Cancer – Gastroesophageal, Pancreatic, and Hepatobiliary |
| A Phase II study to evaluate the efficacy and safety of fruquintinib combined with envafolimab in patients with advanced or unresectable locally advanced osteosarcoma and soft tissue sarcoma | Chenliang Zhou, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China | e23506 Publication Only: Sarcoma |
| Efficacy and safety of surufatinib (Sur) plus paclitaxel (Pac) as second line (2L) treatment for advanced gastric cancer (aGC): Final results from a Phase II trial | Xiuying Xiao, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China | 4028 Poster Session: Gastrointestinal Cancer – Gastroesophageal, Pancreatic, and Hepatobiliary |
| Efficacy and safety of surufatinib (S) plus KN046 (K) and chemotherapy in first line (1L) advanced pancreatic cancer (PC): a single-arm, Phase Ib/II trial | Wenquan Wang, Zhongshan Hospital, Fudan University, Shanghai, China | 4157 Poster Session: Gastrointestinal Cancer – Gastroesophageal, Pancreatic, and Hepatobiliary |
| First-Line Treatment with Surufatinib, Camrelizumab, Nab-paclitaxel, and S-1 in Locally Advanced or Metastatic Pancreatic Ductal Adenocarcinoma (PDAC): A Phase Ib/II Randomized Study | Ru Jia/ Guanghai Dai, the Fifth Medical Center of the PLA General Hospital, Beijing, China | 4161 Poster Session: Gastrointestinal Cancer – Gastroesophageal, Pancreatic, and Hepatobiliary |
| A prospective, single-arm, Phase II trial exploring the use of pamiparib combined with surufatinib as neoadiuvant therapy for advanced, unresectable ovarian cancer (PASSION) | Bairong Xia, The First Affiliated Hospital of University of Science and Technology of China, Hefei, China | 5589 Poster Session: Gynecologic Cancer |
| The efficacy and safety of Surufatinib monotherapy as a third-line treatment for advanced hepatocellular carcinoma: A single-arm, open-label, multi-center Phase II study | Fuxiang Zhou, Zhongnan Hospital of Wuhan University, Wuhan, China | e16209 Publication Only: Gastrointestinal Cancer – Gastroesophageal, Pancreatic, and Hepatobiliary |
| Surufatinib combined with gemcitabine and cisplatin and immune checkpoint inhibitor (ICI) for unresectable locally advanced or metastatic intrahepatic cholangiocarcinoma | Jingtao Zhang/ Xuetao Shi, Cancer Hospital of Shandong First Medical University, Jinan, China | e16222 Publication Only: Gastrointestinal Cancer – Gastroesophageal, Pancreatic, and Hepatobiliary |
| Updated results from a multicenter, single-arm Phase ll study of surufatinib plus sintilimab and lBl310 in patients with high-grade advanced neuroendocrine neoplasm (HG-NEN) | Ming Lu/ Lin Shen, Peking University Cancer Hospital, Beijing, China | e16342 Publication Only: Gastrointestinal Cancer – Gastroesophageal, Pancreatic, and Hepatobiliary |
| A prospective, single-arm, Phase II study of surufatinib in combination with gemcitabine and nab-paclitaxel for the neoadjuvant treatment of resectable and borderline resectable pancreatic cancer | Song Gao/ Jihui Hao, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China | e16442 Publication Only: Gastrointestinal Cancer – Gastroesophageal, Pancreatic, and Hepatobiliary |
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including but not limited to its expectations regarding the therapeutic potential of savolitinib, ranosidenib, fruquintinib and surufatinib, the further clinical development for savolitinib, ranosidenib, fruquintinib and surufatinib, its expectations as to whether any studies on savolitinib, ranosidenib, fruquintinib and surufatinib would meet their primary or secondary endpoints, and its expectations as to the timing of the completion and the release of results from such studies. Such risks and uncertainties include, among other things, assumptions regarding enrollment rates and the timing and availability of subjects meeting a study’s inclusion and exclusion criteria; changes to clinical protocols or regulatory requirements; unexpected adverse events or safety issues; the ability of savolitinib, ranosidenib, fruquintinib and surufatinib, including as combination therapies, to meet the primary or secondary endpoint of a study, to obtain regulatory approval in different jurisdictions and to gain commercial acceptance after obtaining regulatory approval; the potential markets of savolitinib, ranosidenib, fruquintinib and surufatinib for a targeted indication, and the sufficiency of funding. In addition, as certain studies rely on the use of other drug products as combination therapeutics, such risks and uncertainties include assumptions regarding their safety, efficacy, supply and continued regulatory approval. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
CONTACTS
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
HSBC |
Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
Cavendish |
Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
| Date: Thursday, June 05, 2025 |
| Time: 12:50pm EDT (4:50pm GMT / 12:50 am HKT on June 06) |
The Board of Directors of HUTCHMED (China) Limited comprises 9 Directors as follows:
Chairman and Non-executive Director
Dr Dan ELDAR
Executive Directors
Dr Weiguo SU (Chief Executive Officer and Chief Scientific Officer)
Mr CHENG Chig Fung, Johnny (Chief Financial Officer)
Non-executive Directors
Ms Edith SHIH
Ms Ling YANG
Independent Non-executive Directors
Professor MOK Shu Kam, Tony (Senior and Lead Independent Non-executive Director)
Dr Renu BHATIA
Dr Chaohong HU
Mr WONG Tak Wai
The table below provides membership information of the committees on which Board members serve.
| Board Committee | |||||
|---|---|---|---|---|---|
| Directors | Audit Committee | Nomination Committee | Remuneration Committee | Sustainability Committee | Technical Committee |
| Dan ELDAR | – | M | – | – | M |
| Weiguo SU | – | – | – | – | M |
| CHENG Chig Fung, Johnny | – | – | – | M | – |
| Edith SHIH | – | – | M | C | – |
| Ling YANG | – | – | – | – | – |
| MOK Shu Kam, Tony | – | C | – | M | C |
| Renu BHATIA | M | – | C | – | M |
| Chaohong HU | M | M | – | – | M |
| WONG Tak Wai | C | – | M | – | – |
Notes:
C Chairman of the relevant Board committees
M Member of the relevant Board committees
May 14, 2025
Hong Kong, Shanghai, & Florham Park, NJ: Tuesday, May 13, 2025: HUTCHMED (China) Limited (“HUTCHMED” or the “Company”) (Nasdaq/AIM: HCM; HKEX:13) today announces that all ordinary resolutions and special resolution put to its Annual General Meeting (“AGM”) held on May 13, 2025 were duly passed. The poll results of the resolutions were as follows:
| Ordinary Resolutions | Number of Votes (%)* | Passed by Shareholders | |||
| For | Against | Withheld# | |||
| 1. | To consider and adopt the audited Financial Statements, and the Reports of the Directors and the Independent Auditors for the year ended December 31, 2024. | 493,635,784 (99.9863%) |
67,770 (0.0137%) |
54,915 | Yes |
| 2(A). | To re-elect Dr Dan ELDAR as a Director. | 479,808,571 (97.1824%) |
13,910,941 (2.8176%) |
38,955 | Yes |
| 2(B). | To re-elect Dr Weiguo SU as a Director. | 481,283,821 (97.4806%) |
12,438,811 (2.5194%) |
35,835 | Yes |
| 2(C). | To re-elect Mr CHENG Chig Fung, Johnny as a Director. | 481,167,431 (97.4570%) |
12,555,206 (2.5430%) |
35,830 | Yes |
| 2(D). | To re-elect Ms Edith SHIH as a Director. | 466,597,573 (94.5061%) |
27,124,459 (5.4939%) |
35,935 | Yes |
| 2(E). | To re-elect Ms Ling YANG as a Director. | 484,362,585 (98.1042%) |
9,360,052 (1.8958%) |
35,830 | Yes |
| 2(F). | To re-elect Dr Renu BHATIA as a Director. | 493,441,294 (99.9430%) |
281,240 (0.0570%) |
35,935 | Yes |
| 2(G). | To re-elect Dr Chaohong HU as a Director. | 493,441,904 (99.9431%) |
280,740 (0.0569%) |
35,825 | Yes |
| 2(H). | To re-elect Professor MOK Shu Kam, Tony as a Director. | 492,694,533 (99.7918%) |
1,028,006 (0.2082%) |
35,930 | Yes |
| 2(I). | To re-elect Mr WONG Tak Wai as a Director. | 493,633,871 (99.9820%) |
88,768 (0.0180%) |
35,830 | Yes |
| 3. | To re-appoint PricewaterhouseCoopers and PricewaterhouseCoopers Zhong Tian LLP as the Auditors of the Company for Hong Kong financial reporting and US financial reporting purposes, respectively, and to authorize the Directors to fix the Auditors’ remuneration. | 492,729,314 (99.8004%) |
985,390 (0.1996%) |
43,765 | Yes |
| Special Resolution | |||||
| 4. | To grant a general mandate to the Directors to issue additional shares of the Company.^ | 493,550,312 (99.9637%) |
179,327 (0.0363%) |
28,830 | Yes |
| Ordinary Resolution | |||||
| 5. | To grant a general mandate to the Directors to repurchase shares of the Company.^ | 493,321,158 (99.9180%) |
405,036 (0.0820%) |
32,275 | Yes |
* Percentages rounded to 4 decimal places.
# A vote withheld is not a vote in law and is not counted in the calculation of the proportion of the votes for or against a resolution.
^ The full text of Resolutions 4 and 5 are set out in the notice of AGM dated April 8, 2025.
Notes:
(1) All directors of the Company, namely Dr Dan ELDAR, Dr Weiguo SU, Mr CHENG Chig Fung, Johnny, Ms Edith SHIH, Ms Ling YANG, Mr Paul Rutherford CARTER, Dr Renu BHATIA, Dr Chaohong HU, Mr Graeme Allan JACK, Professor MOK Shu Kam, Tony and Mr WONG Tak Wai, attended the AGM, either in person or by means of electronic facilities.
(2) Number of shares entitling the holders to attend and vote on the resolution at the AGM: 871,601,095 shares.
(3) Number of shares entitling the holders to attend and abstain from voting in favor as set out in Rule 13.40 of the Rules Governing the Listing of Securities on The Stock Exchange of Hong Kong Limited (the “Listing Rules”) at the AGM: Nil.
(4) Number of shares for holders required under the Listing Rules to abstain from voting at the AGM: Nil.
(5) The scrutineer for the poll at the AGM was Computershare Investor Services (Jersey) Limited, the Principal Share Registrar of the Company.
About HUTCHMED
HUTCHMED (Nasdaq/AIM: HCM; HKEX: 13) is an innovative, commercial-stage, biopharmaceutical company. Іt is committed to the discovery, global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, and the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
CONTACTS
| Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
| Media Enquiries | |
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
| Panmure Liberum | Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden |
+44 20 7886 2500 |
| HSBC | Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
|
Cavendish |
Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
Hong Kong, Shanghai & Florham Park, NJ — Thursday, April 24, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that new and updated data from several studies of compounds discovered by HUTCHMED including savolitinib, fruquintinib and surufatinib, which will be presented at the upcoming American Association of Cancer Research (AACR) Annual Meeting 2025, taking place on April 25-30, 2025 in Chicago, Illinois.
Details of the presentations are as follows:
| Abstract title | Presenter / Lead author | Presentation details |
| SPONSORED STUDIES | ||
| Targeting KEAP1/NRF2 signaling sensitizes KRAS-driven NSCLC to KRAS inhibitors | Xianwen Yang, HUTCHMED, Shanghai, China | 4450 Poster Session (PO.ET03.04) Tuesday, April 29, 2025 |
| SAVANNAH: Clearance of plasma EGFRm in patients with EGFRm MET-overexpressed (OverExp) and/or -amplified (Amp) NSCLC post-osimertinib (osi) treated with savolitinib (savo) + osi | Jonathan W. Riess, University of California, UC Davis Comprehensive Cancer Center, CA, US | LB416 Late-Breaking Poster Session (LBPO.CL04) Wednesday, April 30, 2025 |
| A phase I open-label positron-emission tomography study to determine brain exposure of [11C]savolitinib in healthy volunteers | Kowser Miah, AstraZeneca, Waltham, MA, US | 4353 Poster Session (PO.ET07.01) Tuesday, April 29, 2025 |
| INVESTIGATOR-INITIATED STUDIES | ||
| A multi-cohort study of treatment regimens for metastatic colorectal cancer (mCRC): Subgroup analysis of sequential therapy between fruquintinib and regorafenib | Wangxia Lv, Cancer Hospital of the University of Chinese Academy of Sciences/ Zhejiang Cancer Hospital, Hangzhou, China | CT085 Poster Session (PO.CT02.03) Monday, April 28, 2025 |
| Phase Ib/II study of fruquintinib (F) combined with capecitabine (C) as maintenance therapy (MT) for RAS/BRAF wild-type metastatic colorectal cancer (mCRC) after first-line treatment with cetuximab combined with chemotherapy | Lin Yang/ Kai Ou, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China | CT222 Poster Session (PO.CT02.02) Tuesday, April 29, 2025 |
| Tyrosine kinase inhibitor (TKI) plus PD-1 blockade in TKI-responsive MSS/pMMR metastatic colorectal adenocarcinoma (mCRC): Results of a multicenter, phase II trial (TRAP) | Jingdong Zhang/ Qian Dong, Liaoning Cancer Hospital & Institute, Cancer Hospital of Dalian University of Technology, Shenyang, China | 6002 Poster Session (PO.CL08.03) Tuesday, April 29, 2025 |
| Efficacy and mechanism of radiotherapy combined with fruquintinib and tislelizumab in mCRC | Xianglin Yuan, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China | 1828 Poster Session (PO.ET08.02) Monday, April 28, 2025 |
| Prediction of surufatinib treatment outcome in advanced grade 3 neuroendocrine tumors (NET G3) patients | Jing Hao, Qilu Hospital of Shandong University, Jinan, China | CT084 Poster Session (PO.CT02.03) Monday, April 28, 2025 |
| Surufatinib and sintilimab in combination with capecitabine for previously treated metastatic small bowel adenocarcinoma or appendiceal carcinoma: A single-arm, single-center, phase Ib/II trial | Yanhong Deng/ Xiaoyu Xie, The Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, China | CT033 Poster Session (PO.CT01.03) Monday, April 28, 2025 |
| GPR34-driven damage-response macrophages underlie therapeutic resistance to surufatinib | Song Gao, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China | 6818 Poster Session (PO.ET07.02) Wednesday, April 30, 2025 |
| Efficacy and mechanism of surufatinib combination with PD-1 monoclonal antibody and chemotherapy for the treatment of pancreatic cancer with liver metastasis in animal models | Guanghai Dai/ Ru Jia, The Fifth Medical Center, Chinese PLA General Hospital, Beijing, China | 6824 Poster Session (PO.ET07.02) Wednesday, April 30, 2025 |
| Efficacy and mechanistic insights of Shengyang Qushi Decoction in managing surufatinib-associated diarrhea | Huangying Tan, China-Japan Friendship Hospital, Beijing, China | 1043 Poster Session (PO.PR02.03) Sunday, April 27, 2025 |
About Lung Cancer
Lung cancer is the leading cause of cancer death, accounting for about one-fifth of all cancer deaths.[1] More than a third of the world’s lung cancer patients are in China. Lung cancer is broadly split into non-small cell lung cancer (“NSCLC”) and small cell lung cancer, with NSCLC accounting for about 80% of cases.[2] Approximately 10-15% of NSCLC patients in the US and Europe and 30-40% of patients in Asia have an epidermal growth factor receptor (“EGFR”) mutation (“EGFRm”).[3],[4],[5],[6] Approximately 2-3% of NSCLC patients have tumors with MET exon 14 skipping alterations, a targetable mutation in the MET gene.[7]
About Savolitinib and MET Aberrations in Lung Cancer
Savolitinib is an oral, potent, and highly selective MET tyrosine kinase inhibitor (“TKI”) being jointly developed by AstraZeneca and HUTCHMED and commercialized by AstraZeneca. MET is a tyrosine kinase receptor that has an essential role in normal cell development.[8] Savolitinib blocks atypical activation of the MET receptor tyrosine kinase pathway that occurs because of mutations (such as exon 14 skipping alterations or other point mutations), gene amplification or protein overexpression. MET overexpression and/or amplification can lead to tumor growth and the metastatic progression of cancer cells, and is a known mechanism of acquired resistance to EGFR TKIs.[8],[9] The prevalence of MET depends on the sample type, detection method and assay cut-off used.
Savolitinib is approved in China and is marketed under the brand name ORPATHYS® by AstraZeneca for the treatment of adult patients with locally advanced or metastatic NSCLC with MET exon 14 skipping alteration, representing the first selective MET inhibitor approved in China. It is currently under clinical development for multiple tumor types, including lung, kidney, and gastric cancers as a single treatment and in combination with other medicines.
About Savolitinib in combination with TAGRISSO® (osimertinib)
Among patients who experience disease progression following treatment with a third-generation EGFR TKI, approximately 15-50% present with MET aberration, depending on the sample type, detection method and assay cut-off used. TAGRISSO® is a third-generation, irreversible EGFR-TKI with proven clinical activity in NSCLC, including against central nervous system metastases. Treatment with savolitinib in combination with TAGRISSO® has been studied extensively in these patients in the TATTON (NCT02143466) and SAVANNAH (NCT03778229) studies. The encouraging results led to the initiation of several Phase III trials in this setting including the SACHI trial in China (NCT05015608) and the global SAFFRON trial (NCT05261399), as well as the SANOVO trial in China (NCT05009836).
This combination represents a promising chemo-free oral treatment strategy to address mechanisms of resistance in this advanced setting. Positive data from the SACHI randomized Phase III trial has led to the filing of a second New Drug Application (“NDA”) in China. Strong data from the SAVANNAH single-arm Phase II study was recently presented at the European Lung Cancer Congress (ELCC) in March 2025 demonstrated high, clinically meaningful and durable objective response rate (ORR), with consistent safety results. The SAFFRON randomized Phase III trial is progressing. Following AstraZeneca’s consultation with the US Food and Drug Administration (“FDA”), we look forward to completing the SAFFRON trial as soon as possible to support potential US and other global registration filings.
SACHI: The SACHI China Phase III trial met the primary endpoint of progression free survival (PFS) during its interim analysis towards the end of 2024 and a NDA was accepted and granted Breakthrough Therapy Designation and Priority Review status in China in December 2024. SACHI evaluated the combination of savolitinib and TAGRISSO® for the treatment of patients with EGFRm, MET-amplified locally advanced or metastatic NSCLC after progression on EGFR TKI compared to platinum-based doublet chemotherapy. Results will be presented at an upcoming scientific conference.
SAFFRON: In 2023, savolitinib and TAGRISSO® received Fast Track Designation from the US FDA in this setting. The global SAFFRON Phase III trial is currently ongoing to assess the savolitinib plus TAGRISSO® combination versus platinum-based doublet chemotherapy in patients with EGFRm, MET-overexpressed and/or amplified, locally advanced or metastatic NSCLC following progression on treatment with TAGRISSO®. Patients are being prospectively selected using the high MET level cut-off identified in SAVANNAH.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including but not limited to its expectations regarding the therapeutic potential of fruquintinib, savolitinib and surufatinib, the further clinical development for fruquintinib, savolitinib and surufatinib, its expectations as to whether any studies on fruquintinib, savolitinib and surufatinib would meet their primary or secondary endpoints, and its expectations as to the timing of the completion and the release of results from such studies. Such risks and uncertainties include, among other things, assumptions regarding enrollment rates and the timing and availability of subjects meeting a study’s inclusion and exclusion criteria; changes to clinical protocols or regulatory requirements; unexpected adverse events or safety issues; the ability of fruquintinib, savolitinib and surufatinib, including as combination therapies, to meet the primary or secondary endpoint of a study, to obtain regulatory approval in different jurisdictions and to gain commercial acceptance after obtaining regulatory approval; the potential markets of fruquintinib, savolitinib and surufatinib for a targeted indication, and the sufficiency of funding. In addition, as certain studies rely on the use of osimertinib, capecitabine, sintilimab or tislelizumab, as combination therapeutics, such risks and uncertainties include assumptions regarding their safety, efficacy, supply and continued regulatory approval. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
CONTACTS
| Investor Enquiries | +852 2121 8200 / ir@hutch-med.com |
| Media Enquiries | |
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
| Panmure Liberum | Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
| HSBC | Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
| Cavendish | Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
REFERENCE
[1] World Health Organization. International Agency for Research on Cancer. All cancers fact sheet. Available at: https://gco.iarc.who.int/media/globocan/factsheets/cancers/39-all-cancers-fact-sheet.pdf. Accessed November 2022.
[2] American Cancer Society. What is Lung Cancer? Available at: https://www.cancer.org/cancer/types/lung-cancer/about/what-is.html. Accessed November 2022.
[3] Knight SB, et al. Progress and prospects of early detection in lung cancer. Open Biol. 2017;7(9): 170070. DOI: 10.1098/RSOB.170070.
[4] Keedy VL, et al. American Society of Clinical Oncology Provisional Clinical Opinion: Epidermal Growth Factor Receptor (EGFR) Mutation Testing for Patients with Advanced Non-Small-Cell Lung Cancer Considering First-Line EGFR Tyrosine Kinase Inhibitor Therapy. J Clin Oncol. 2011:29;2121-27. DOI: 10.1200/JCO.2010.31.8923.
[5] Zhang Y, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7(48). DOI: 10.18632/oncotarget.12587.
[6] Szumera-Ciećkiewicz A, et al. EGFR Mutation Testing on Cytological and Histological Samples in 11. Non-Small Cell Lung Cancer: a Polish, Single Institution Study and Systematic Review of European Incidence. Int J Clin Exp Pathol. 2013:6;2800-12. PMID: 24294366.
[7] Vuong HG, et al. Clinicopathological implications of MET exon 14 mutations in non-small cell lung cancer – A systematic review and meta-analysis. Lung Cancer. 2018; 123: 76-82. DOI: 10.1016/j.lungcan.2018.07.006.
[8] Uchikawa E, et al. Structural basis of the activation of c-MET receptor. Nat Commun. 2021;12(4074). DOI: 10.1038/s41467-021-24367-3.
[9] Wang Q, et al. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. Journal of Hematology & Oncology. 2019;63. DOI: 10.1186/s13045-019-0759-9.
Hong Kong, Shanghai & Florham Park, NJ — Tuesday, April 22, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that it has completed enrollment of the registration phase of its Phase II trial of savolitinib in gastric cancer patients with MET amplification.
This clinical trial is a single-arm, multi-center, open-label, Phase II registration study to evaluate the efficacy, safety and tolerability of savolitinib in treating gastric cancer or gastroesophageal junction (“GEJ”) adenocarcinoma patients with MET amplification. Primary endpoint is objective response rate (“ORR”) evaluated by the Independent Review Committee (“IRC”) (RECIST 1.1). Secondary endpoints include progression free survival (PFS) and incidence of various adverse events (AE), among others. A total of 64 patients have been enrolled in the study. Further details may be found at clinicaltrials.gov using identifier NCT04923932.
As reported at the American Association for Cancer Research Annual Meeting, interim results from the study showed a 45% ORR confirmed by IRC and a 50% ORR in patients with high MET gene copy number. 4‑month duration of response (DOR) rate was 85.7% with median follow up time of 5.5 months. The most common grade 3 or higher treatment-related adverse events (“TRAE”) (≥ 5%) were platelet count decreased, hypersensitivity, anemia, neutropenia and hepatic function abnormal. Only one patient discontinued treatment due to grade 4 liver function abnormal (TRAE) and no patient died due to TRAE.
The China’s National Medical Products Administration (“NMPA”) has granted Breakthrough Therapy Designation to savolitinib for the treatment of locally advanced or metastatic gastric cancer or GEJ adenocarcinoma patients with MET amplification who have failed at least two lines of standard therapies. If positive, HUTCHMED may initiate plans to apply for marketing authorization of savolitinib for gastric cancer in China in late 2025.
About Gastric Cancer with MET Amplification
MET-driven gastric cancer has a very poor prognosis.[1] It is estimated that MET amplification accounts for approximately 4-6% of gastric cancer patients.[2],[3] The annual incidence of MET amplification gastric cancer is estimated to be approximately 18,000 in China.[4] The ongoing registration trial follows multiple Phase II studies conducted in Asia to study savolitinib in MET-driven gastric cancer patients, including VIKTORY.[3] The VIKTORY study reported a 50% ORR in patients whose tumors harbored MET amplification and were treated with savolitinib monotherapy.
About Savolitinib
Savolitinib is an oral, potent, and highly selective MET tyrosine kinase inhibitor (“TKI”) being jointly developed by AstraZeneca and HUTCHMED and commercialized by AstraZeneca. MET is a tyrosine kinase receptor that has an essential role in normal cell development.[5] Savolitinib blocks atypical activation of the MET receptor tyrosine kinase pathway that occurs because of mutations (such as exon 14 skipping alterations or other point mutations), gene amplification or protein overexpression.
Savolitinib is approved in China and is marketed under the brand name ORPATHYS® by our partner, AstraZeneca, for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with MET exon 14 skipping alteration, representing the first selective MET inhibitor approved in China. It has been included in the National Reimbursement Drug List of China (NRDL) since March 2023.
It is currently under clinical development for multiple tumor types, including lung, kidney, and gastric cancers as a single treatment and in combination with other medicines.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the therapeutic potential of savolitinib, the further clinical development for savolitinib, its expectations as to whether any studies on savolitinib would meet their primary or secondary endpoints, and its expectations as to the timing of the completion and the release of results from such studies. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding enrollment rates and the timing and availability of subjects meeting a study’s inclusion and exclusion criteria; changes to clinical protocols or regulatory requirements; unexpected adverse events or safety issues; the ability of savolitinib, including as a combination therapy, to meet the primary or secondary endpoint of a study, to obtain regulatory approval in different jurisdictions and to gain commercial acceptance after obtaining regulatory approval; the potential market of savolitinib for a targeted indication and the sufficiency of funding. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
[1] Catenacci DV, Ang A, Liao WL, et al. MET tyrosine kinase receptor expression and amplification as prognostic biomarkers of survival in gastroesophageal adenocarcinoma. Cancer. 2017;123(6):1061-1070. doi:10.1002/cncr.30437
[2] Lee J, Kim ST, Kim K, et al. Tumor Genomic Profiling Guides Patients with Metastatic Gastric Cancer to Targeted Treatment: The VIKTORY Umbrella Trial. Cancer Discov. 2019;9(10):1388-1405. doi:10.1158/2159-8290.CD-19-044
[3] Van Cutsem E, Karaszewska B, Kang YK, et al. A Multicenter Phase II Study of AMG 337 in Patients with MET-Amplified Gastric/Gastroesophageal Junction/Esophageal Adenocarcinoma and Other MET-Amplified Solid Tumors. Clin Cancer Res. 2019;25(8):2414-2423. doi:10.1158/1078-0432.CCR-18-1337
[4] Global Cancer Observatory. China Fact Sheet. https://gco.iarc.who.int/media/globocan/factsheets/populations/160-china-fact-sheet.pdf. Accessed April 7, 2025.
[5] Uchikawa E, et al. Structural basis of the activation of c-MET receptor. Nat Commun. 2021;12(4074).
| Date: Monday, June 09, 2025 |
| Time: 2:40 pm EDT (6:40pm GMT / 2:40 am HKT on June 10) |
| Date: Wednesday, May 14, 2025 |
| Time: 11:20am PDT (6:20pm GMT / 2:20am HKT on May 15) |
| Date: Tuesday, May 13, 2025 |
| Time: 4pm Hong Kong Time (9am London Time) |
| Location:1st Floor, Harbour Grand Kowloon, 20 Tak Fung Street, Hung Hom, Kowloon, Hong Kong |
Hong Kong, Shanghai, & Florham Park, NJ — Monday, April 7, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM: HCM; HKEX: 13) today announces that its 2024 Annual Report, together with the Notice of Annual General Meeting and the Form of Proxy (“AGM Materials”), will be posted on April 8, 2025 to those shareholders who have elected to receive the AGM Materials in printed form. The documents can also be accessed from the HUTCHMED website (www.hutch-med.com).
The 2025 Annual General Meeting (“AGM”) will be an electronic/hybrid meeting to be held at 1st Floor, Harbour Grand Kowloon, 20 Tak Fung Street, Hung Hom, Kowloon, Hong Kong on Tuesday, May 13, 2025 at 4:00 pm Hong Kong Time (9:00 am London Time), with online access through an online platform as detailed in the AGM Materials.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery, global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception, HUTCHMED has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
CONTACTS
| Investor Enquiries | +852 2121 8200 / ir@hutch-med.com |
| Media Enquiries | |
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
| Panmure Liberum | Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
| HSBC | Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
| Cavendish | Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
Hong Kong, Shanghai, & Florham Park, NJ: Monday, March 31, 2025: HUTCHMED (China) Limited (“HUTCHMED” or the “Company”) (Nasdaq/AIM: HCM; HKEX:13) today announces that the ordinary resolution put to its Extraordinary General Meeting (“EGM”) held on March 31, 2025 was duly passed.
Reference is made to the notice of EGM dated March 14, 2025 and the circular to shareholders dated March 14, 2025 (the “Circular”) issued by the Company. Unless otherwise defined herein, capitalized terms used in this announcement shall have the same meanings as those defined in the Circular.
The poll results of the ordinary resolution were as follows:
| Ordinary Resolution | Number of Votes (%)* | Passed by Shareholders | ||
| For | Against | Withheld# | ||
| To approve the transactions related to the sale and purchase of a total of 45% equity interest in Shanghai Hutchison Pharmaceuticals Limited under various agreements, and all actions by the Company and/or its subsidiaries pursuant or incidental to such transactions.^ |
475,229,253 (99.9745%) |
121,270 (0.0255%) |
1,670 | Yes |
* Percentages rounded to 4 decimal places.
# A vote withheld is not a vote in law and is not counted in the calculation of the proportion of the votes for or against a resolution.
^ The full text of the resolution is set out in the notice of EGM dated March 14, 2025.
Notes:
- Except for Ms Ling YANG who had prior overseas commitments and was unable to attend the EGM, all directors of the Company, namely Dr Dan ELDAR, Dr Weiguo SU, Mr CHENG Chig Fung, Johnny, Ms Edith SHIH, Mr Paul Rutherford CARTER, Dr Renu BHATIA, Dr Chaohong HU, Mr Graeme Allan JACK, Professor MOK Shu Kam, Tony and Mr WONG Tak Wai, attended the EGM, either in person or by means of electronic facilities.
- Number of shares entitling the holders to attend and vote on the resolution at the EGM: 871,601,095 shares.
- Number of shares entitling the holders to attend and abstain from voting in favor as set out in Rule 13.40 of the Rules Governing the Listing of Securities on The Stock Exchange of Hong Kong Limited (the “Listing Rules”) at the EGM: Nil.
- Number of shares for holders required under the Listing Rules to abstain from voting at the EGM: Nil.
- The scrutineer for the poll at the EGM was Computershare Investor Services (Jersey) Limited, the Principal Share Registrar of the Company.
About HUTCHMED
HUTCHMED (Nasdaq/AIM: HCM; HKEX: 13) is an innovative, commercial-stage, biopharmaceutical company. Іt is committed to the discovery, global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, and the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
CONTACTS
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
| HSBC | Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
| Cavendish | Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
— First and only EZH2 inhibitor approved by the NMPA —
—HUTCHMED’s fourth product, and its first approval in hematological malignancies —
Hong Kong, Shanghai & Florham Park, NJ — Friday, March 21, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that the New Drug Application (“NDA”) for TAZVERIK® (tazemetostat) has been granted conditional approval in China for the treatment of adult patients with relapsed or refractory (“R/R”) follicular lymphoma (“FL”) with EZH2 mutation who have received at least two prior systemic therapies. This approval follows the priority review status by the National Medical Products Administration (“NMPA”) and marks the first nationwide regulatory approval for TAZVERIK® in China.
The conditional approval by the NMPA was supported by results from a multicenter, open-label, Phase II bridging study in China, and clinical studies conducted by Epizyme, Inc. (“Epizyme”), an Ipsen company, outside China. The primary objective of the bridging study is to evaluate the objective response rate (“ORR”) of TAZVERIK® for the treatment of patients with R/R FL whose disease harbor EZH2 mutations. The secondary objectives included duration of response (“DoR”), progression-free survival (PFS), and overall survival (OS) of TAZVERIK® for the treatment of R/R FL patients, as well as to evaluate the safety and pharmacokinetics. Additional details can be found at clinicaltrials.gov, using identifier NCT05467943.
“This approval represents a significant advancement in the management of this challenging disease. The majority of FL patients experience multiple relapses over their lifetime, posing substantial treatment difficulties and often leading to poor outcomes,” said Dr Junning Cao of Fudan University Cancer Center and the lead principal investigator of the bridging study. “TAZVERIK® has demonstrated promising efficacy in patients harboring EZH2 mutation in clinical trials. We are eager to provide this transformational epigenetic therapy to patients in China who have long sought new effective treatment options.”
“We are thrilled to be able to bring this innovative EZH2 inhibitor to patients in China. This approval highlights our dedication to addressing unmet medical needs not only through our internal pipeline, but also through partnering,” said Dr Michael Shi, Head of R&D and Chief Medical Officer of HUTCHMED. “It also marks our first approval in hematological malignancies, unveiling a new chapter for HUTCHMED as we extend our footprint into this disease area. As we move forward, we are dedicated to making this product available to R/R FL patients as soon as possible and will continue striving to make a meaningful impact on the lives of more patients suffering from devastating diseases.”
TAZVERIK® is a first-in-class methyltransferase inhibitor of EZH2 developed by Epizyme. It is approved by the US Food and Drug Administration (“FDA”) for the treatment of certain patients with R/R FL and certain patients with advanced epithelioid sarcoma (“ES”) under the FDA accelerated approval program. It is also approved by the Japan Ministry of Health, Labour and Welfare (MHLW) for certain patients with R/R FL. In 2021, HUTCHMED and Epizyme entered a strategic partnership. HUTCHMED is responsible for the research, development, manufacturing and commercialization of TAZVERIK® in China Mainland, Hong Kong, Macau and Taiwan. Epizyme will be the Marketing Authorization Holder of TAZVERIK® in China.
TAZVERIK® was approved for use in the Hainan Boao Lecheng International Medical Tourism Pilot Zone (Hainan Pilot Zone) in May 2022, under the Clinically Urgently Needed Imported Drugs scheme, for the treatment of certain patients with ES and FL consistent with the label as approved by the FDA. TAZVERIK® was approved in Macau in March 2023 and in Hong Kong in May 2024.
The ongoing SYMPHONY-1 study will serve as the confirmatory trial to validate the clinical benefits of TAZVERIK®. SYMPHONY-1 is an international, multicenter, randomized, double-blind, active-controlled, 3-stage, biomarker-enriched, confirmatory Phase Ib/III study, which is designed to evaluate the safety and efficacy of TAZVERIK® in combination with rituximab and lenalidomide (R²) in patients with R/R FL after at least one prior line of therapy (NCT04224493). Epizyme is the sponsor of SYMPHONY-1 and HUTCHMED is leading the study in China.
About Follicular Lymphoma
FL is the second most common subtype of non-Hodgkin’s lymphoma (“NHL”), making up 20-30% of all NHL. In 2022, there were an estimated 81,000 and 78,000 new cases of NHL in China and the US, respectively.[1],[2],[3]
About Tazemetostat approval in the United States and Japan
Tazemetostat is a methyltransferase inhibitor indicated in the United States for the treatment of:
- Adults and pediatric patients aged 16 years and older with metastatic or locally advanced ES not eligible for complete resection.
- Adult patients with R/R FL whose tumors are positive for an EZH2 mutation as detected by an FDA-approved test and who have received at least two prior systemic therapies.
- Adult patients with R/R FL who have no satisfactory alternative treatment options.
These indications are approved under accelerated approval by the US FDA based on ORR and DoR. Continued approval for these indications may be contingent upon verification and description of clinical benefit in confirmatory trials.
The most common (≥20%) adverse reactions in patients with ES are pain, fatigue, nausea, decreased appetite, vomiting and constipation. The most common (≥20%) adverse reactions in patients with FL are fatigue, upper respiratory tract infection, musculoskeletal pain, nausea and abdominal pain.
Please see the US Full Prescribing Information for TAZVERIK® (tazemetostat).
TAZVERIK® is approved in Japan with the indication of relapsed or refractory EZH2 gene mutation-positive FL (only when standard treatment is not applicable).
TAZVERIK® is commercialized by Epizyme in the US and by Eisai in Japan.
TAZVERIK® is a registered trademark of Epizyme Inc., an Ipsen company.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the therapeutic potential of tazemetostat for the treatment of patients with relapsed or refractory follicular lymphoma and the further clinical development of tazemetostat in this and other indications. Forward‑looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding the sufficiency of clinical data to support NDA approval of tazemetostat for the treatment of patients with follicular lymphoma in China and other jurisdictions, the safety profile of tazemetostat, HUTCHMED’s ability to fund, implement and complete its further clinical development and commercialization plans for tazemetostat, and the timing of these events. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
CONTACTS
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
HSBC |
Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
Cavendish |
Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
[1] NCCN Guidelines for Patients: Follicular Lymphoma. https://www.nccn.org/patients/guidelines/content/PDF/nhl-follicular-patient.pdf. Accessed February 17, 2025.
[2] SEER Cancer Stat Facts: Follicular Lymphoma. National Cancer Institute. https://seer.cancer.gov/statfacts/html/follicular.html. Accessed February 17, 2025
[3] The Global Cancer Observatory (GLOBOCAN), Cancer Today | IARC. https://gco.iarc.who.int/today/en/dataviz/bars?cancers=34. Accessed February 17, 2025.
Hong Kong, Shanghai & Florham Park, NJ — Thursday, March 20, 2025: HUTCHMED (China) Limited (“HUTCHMED” or the “Company”) (Nasdaq/AIM:HCM, HKEX:13) today announces that Mr Paul Rutherford Carter and Mr Graeme Allan Jack, who have both served as Independent Non-executive Directors of the Company for more than eight years, have informed the Company that they would not seek re-election after retiring from the Board at the forthcoming annual general meeting of the Company to be held on May 13, 2025 (“AGM”). Consequently, both will cease to be Independent Non-executive Directors of the Company at the conclusion of the AGM. Upon their retirement, they will also step down from their roles as chairmen and members of the board committees of the Company.
In connection with the intended retirement of the above Directors, the Board has approved the following changes to the composition of the board committees and Senior and Lead Independent Non-executive Director of the Company, effective from the conclusion of the AGM, subject to the respective Directors being re-elected as Directors by the shareholders at the AGM:-
- Professor Mok Shu Kam, Tony will be appointed as Senior and Lead Independent Non-executive Director;
- Mr Wong Tak Wai will be appointed as the chairman of the Audit Committee and a member of the Remuneration Committee;
- Dr Chaohong Hu will be appointed as a member of the Audit Committee; and
- Dr Renu Bhatia will be appointed as the chairman of the Remuneration Committee.
Each of Professor Mok, Mr Wong, Dr Hu and Dr Bhatia is currently an Independent Non-executive Director of the Company.
Dr Dan Eldar, the Chairman of HUTCHMED, said “Mr Carter, who has served as the Senior Independent Non-executive Director and the chairman of the Remuneration Committee, has played a pivotal role in shaping the remuneration policies and practices of the Company. His leadership and guidance have been crucial in retaining and motivating a broader and more diverse pool of employees of the highest caliber and experience needed to shape and execute the strategy of the Company. The Board extends its appreciation to Mr Carter for his outstanding service and contributions to the success of the Company.”
Dr Eldar continued, “Mr Jack, who has served as the chairman of the Audit Committee, has been instrumental in overseeing the financial reporting and audit processes of the Company, ensuring the highest standards of integrity and transparency. The Board expresses its deepest gratitude to Mr Jack for his invaluable contributions and dedication to the Company. We wish them both all the best in their future endeavors.”
Pursuant to the requirements of Rule 13.51(2) of the HK Listing Rules, each of Mr Carter and Mr Jack have confirmed that he has no disagreement with the Board and that there are no other matters that need to be brought to the attention of the shareholders of the Company in connection with his retirement from the Board.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery, global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, and the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This announcement contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, the risk that current or future appointees to HUTCHMED’s board of directors are not effective in their respective positions, the difficulty in locating and recruiting suitable candidates for its board of directors and the management difficulties which may arise from changes in HUTCHMED’s board of directors. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, on AІM and with The Stock Exchange of Hong Kong Limited. HUTCHMED undertakes no obligation to update or revise the information contained in this announcement, whether as a result of new information, future events or circumstances or otherwise.
CONTACTS
| Investor Enquiries | +852 2121 8200 / ir@hutch-med.com |
| Media Enquiries | |
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
| Panmure Liberum | Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
| HSBC | Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
| Cavendish | Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
— SAVANNAH Phase II trial demonstrated high and durable response rates with savolitinib plus TAGRISSO® in MET-high lung cancer, representing a promising chemo-free oral treatment strategy to address mechanisms of resistance in the advanced setting —
— Long-term survival benefit and safety observed in savolitinib Phase IIIb study in METex14 NSCLC —
Hong Kong, Shanghai & Florham Park, NJ — Thursday, March 20, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that new and updated data from several studies of compounds discovered by HUTCHMED, savolitinib and surufatinib, will be presented at the European Lung Cancer Congress (ELCC) 2025, taking place on March 26-29, 2025 in Paris, France.
| Title: | SAVANNAH: Savolitinib (savo) + osimertinib (osi) in patients (pts) with EGFRm advanced NSCLC and MET overexpression (OverExp) and/or amplification (Amp) following progressive disease (PD) on osi |
| Lead Author: | Myung-Ju Ahn, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea |
| Session: | Proffered Paper session 1 |
| Abstract Number: | #2O |
| Date & Time: | Wednesday, March 26, 2025,16:45 Central European Time |
| Location: | South Paris Room |
Results from the SAVANNAH Phase II trial (NCT03778229) showed savolitinib (300mg BID[1]) plus TAGRISSO® demonstrated a clinically meaningful and durable objective response rate (“ORR”) in patients with epidermal growth factor receptor mutated (“EGFRm”) non-small cell lung cancer (“NSCLC”) with high levels of MET overexpression and/or amplification whose disease progressed on treatment with first line TAGRISSO®.
Savolitinib plus TAGRISSO® demonstrated confirmed ORR of 56% (95% CI[2]: 45%–67%) and 55% (95% CI: 43%–66%), median duration of response (“DoR”) of 7.1 (95% CI: 5.6–9.6) and 9.9 (95% CI: 6.0–13.7) months, and median progression-free survival (“PFS”) of 7.4 (95% CI: 5.5–7.6) and 7.5 (95% CI: 6.4–11.3) months by investigator and blinded independent central review (BICR) assessment, respectively.
Safety results and discontinuation rates due to adverse events were consistent with the established profiles of each medicine and no new safety concerns were reported. In all patients treated with savolitinib (300mg BID) plus TAGRISSO®, Grade 3 or higher adverse events (AEs) occurred in 57% and Grade 3 or higher treatment related adverse events (TRAEs) occurred in 32% of the patients.
Savolitinib is an oral, potent and highly selective MET tyrosine kinase inhibitor (“TKI”) being jointly developed and commercialized by AstraZeneca and HUTCHMED. In 2023, savolitinib and TAGRISSO® received Fast Track Designation from the US Food and Drug Administration (FDA) in this setting.
| Title: | Final Overall Survival and Long-term Safety Outcomes of Savolitinib in Patients with Locally Advanced or Metastatic NSCLC Harboring MET Exon 14 (METex14) Mutation: An Update from a Phase 3b Study |
| Lead Author: | Yongfeng Yu, Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China |
| Session: | Poster Display session |
| Abstract Number: | #80P |
| Date & Time: | Friday March 28, 2025,13:00 Central European Time |
| Location: | Poster area |
Updated results from the savolitinib Phase IIIb study in China demonstrated survival benefits and long-term safety in MET exon 14 skipping alteration NSCLC, particularly in treatment-naïve patients (NCT04923945). Among the 166 patients who received savolitinib treatment, the median follow-ups for 87 treatment-naïve patients and 79 previously treated patients were 34.5 and 25.1 months, respectively. In 87 treatment-naïve patients, median overall survival (“OS”) was 28.3 months (95% CI: 17.5–not evaluable), and the 36-month OS rate was 44.7%. In 79 previously treated patients, median OS was 25.3 months (95% CI: 20.5–30.5), and the 24-month OS rate was 51.7%. Post-hoc OS subgroup analysis suggested that patients with baseline brain metastasis also gain survival benefit with median OS of 15.3 months and 25.3 months in treatment-naïve patients (10/87 patients) and previously treated (21/79 patients) with brain metastasis, respectively. No new safety signal was observed.
Savolitinib is approved in China under the brand name ORPATHYS® for this patient population.
| Title: | Final overall survival (OS) of surufatinib plus PD-1/PD-L1 antibodies as maintenance therapy following first line (1L) platinum-based chemotherapy (Chemo) plus PD-1/PD-L1 antibodies in patients (pts) with extensive-stage small cell lung cancer (ES-SCLC) |
| Lead Author: | Yi Hu, Chinese PLA General Hospital, Beijing, China |
| Session: | Poster Display session |
| Abstract Number: | #310P |
| Date & Time: | Friday March 28, 2025,13:00 Central European Time |
| Location: | Poster area |
Results from the exploratory study suggests surufatinib plus immunotherapy as maintenance therapy following first line chemo‑immunotherapy demonstrated durable survival benefit for patients with extensive-stage small cell lung cancer (“SCLC”) patients (NCT05509699). At data cut-off on July 31, 2024, a total of 21 patients were enrolled in this single arm Phase IIa part of the study and received at least one dose of surufatinib plus PD‑1/PD‑L1 antibodies treatment. The median follow-up duration was 17.1 months for maintenance and 22.5 months for first line (induction + maintenance) therapy. The 12-month and 18-month OS rates were both 57.1% for maintenance therapy; and 85.7% and 57.1% for first line therapy.
About NSCLC and SAVANNAH
Lung cancer is the leading cause of cancer death among men and women, accounting for about one-fifth of all cancer deaths. Lung cancer is broadly split into SCLC or NSCLC, the latter accounting for about 80% of cases. Approximately 10 to 15% of patients with NSCLC in the US and Europe and 30 to 40% of patients in Asia have an epidermal growth factor receptor (“EGFR”) mutation. While EGFR TKIs have significantly improved outcomes in the first line setting, treatment resistance and disease progression are extremely common, and a significant unmet need exists in later-line settings for effective and well-tolerated treatment options.
SAVANNAH is an ongoing randomized, global Phase II trial studying the efficacy of savolitinib added to TAGRISSO® in patients with EGFRm, locally advanced or metastatic NSCLC with MET overexpression and/or amplification who progressed following treatment with TAGRISSO®. Based on the original single-arm trial design, patients were treated with savolitinib 300 or 600mg QD[3] or 300mg BID, in combination with oral TAGRISSO® 80mg QD. In 2022, a comparison of savolitinib 300 mg BID and TAGRISSO® 80 mg QD to savolitinib 300mg BID and placebo was added to the trial to evaluate contribution of components.
The trial enrolled over 360 patients in more than 80 centers globally, including in North America, Europe, South America and Asia. The primary endpoint is ORR and key secondary endpoints include PFS and DoR.
In August 2022, positive interim results from the SAVANNAH trial were presented at the International Association for the Study of Lung Cancer 2022 World Conference on Lung Cancer (WCLC).
The global SAFFRON Phase III trial is currently ongoing to further assess the savolitinib plus TAGRISSO® combination versus platinum-based doublet chemotherapy in patients with EGFRm, MET-overexpressed and/or amplified, locally advanced or metastatic NSCLC following TAGRISSO®. Patients are being prospectively selected using the high MET level cut-off identified in SAVANNAH.
About Savolitinib
Savolitinib is an oral, potent, and highly selective MET TKI that has demonstrated clinical activity in advanced solid tumors. MET is a tyrosine kinase receptor that has an essential role in normal cell development. Savolitinib blocks atypical activation of the MET receptor tyrosine kinase pathway that occurs because of mutations (such as exon 14 skipping alterations or other point mutations), gene amplification or protein overexpression. MET overexpression and/or amplification can lead to tumor growth and the metastatic progression of cancer cells, and is a known mechanism of acquired resistance to EGFR TKIs. The prevalence of MET depends on the sample type, detection method and assay cut-off used.
Savolitinib is approved in China and is marketed under the brand name ORPATHYS® by our partner, AstraZeneca, for the treatment of adult patients with locally advanced or metastatic NSCLC with MET exon 14 skipping alteration, representing the first selective MET inhibitor approved in China. It has been included in the National Reimbursement Drug List of China (“NRDL”) since March 2023.
It is currently under clinical development for multiple tumor types, including lung, kidney, and gastric cancers as a single treatment and in combination with other medicines.
About Surufatinib
Surufatinib is a novel, oral angio-immuno kinase inhibitor that selectively inhibits the tyrosine kinase activity associated with vascular endothelial growth factor receptors (VEGFRs) and fibroblast growth factor receptor (FGFR), which both inhibit angiogenesis, and colony stimulating factor-1 receptor (CSF-1R), which regulates tumor-associated macrophages, promoting the body’s immune response against tumor cells. Its unique dual mechanism of action may be very suitable for possible combinations with other immunotherapies, where there may be synergistic anti-tumor effects.
Surufatinib is marketed in China by HUTCHMED under the brand name SULANDA®, and was first included in the NRDL in January 2022 for the treatment of non-pancreatic and pancreatic neuroendocrine tumors (NETs).
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery, global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception, it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, and the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit www.hutch‑med.com or follow us on LinkedIn.
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the therapeutic potential of the savolitinib or surufatinib for the treatment of patients with lung cancer and the further clinical development of the savolitinib or surufatinib in this and other indications. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding the timing and outcome of clinical studies and the sufficiency of clinical data to support New Drug Application approval of the savolitinib or surufatinib in lung cancer or other indications in the US, China or other jurisdictions, its potential to gain approvals from regulatory authorities on an expedited basis or at all, the safety profile of savolitinib or surufatinib, HUTCHMED’s ability to fund, implement and complete its further clinical development and commercialization plans for savolitinib or surufatinib and the timing of these events. In addition, as certain studies rely on the use of other drug products such as osimertinib as combination therapeutics with savolitinib and PD-1/PD-L1 antibodies as combination therapeutics with surufatinib, such risks and uncertainties include assumptions regarding the safety, efficacy, supply and continued regulatory approval of these therapeutics. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the U.S. Securities and Exchange Commission, on AIM and on The Stock Exchange of Hong Kong Limited. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
CONTACTS
| Investor Enquiries | +852 2121 8200 / ir@hutch-med.com |
| Media Enquiries | |
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
| Panmure Liberum | Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
| HSBC | Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
| Cavendish | Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
REFERENENCES
[1] BID = Twice per day.
[2] CI = Confidence interval.
[3] QD = Once per day.
65% oncology products revenue growth drove profitable operation and supported new ATTC platform
Hong Kong, Shanghai & Florham Park, NJ — Wednesday, March 19, 2025: HUTCHMED (China) Limited (“HUTCHMED”, the “Company” or “we”) (HKEX:13; Nasdaq/AIM:HCM) today reports its financial results for the year ended December 31, 2024 and provides updates on key clinical and commercial developments.
HUTCHMED to host results webcasts today at 8:00 a.m. EDT / 12:00 noon GMT / 8:00 p.m. HKT in English on Wednesday, March 19, 2025, and tomorrow at 8:30 a.m. HKT in Chinese (Putonghua) on Thursday, March 20, 2025. After registration, investors may access the live webcast via HUTCHMED’s website at www.hutch-med.com/event.
All amounts are expressed in US dollars unless otherwise stated.
Global commercial progress and delivery of sustainable growth
- FRUZAQLA® (fruquintinib) ex-China in-market sales1 of $290.6 million in 2024 by Takeda, sustaining momentum in its first full year driven by rapid US patient uptake, and EU and Japan launches, triggering a sales milestone from Takeda2. Total oncology products in-market sales up 134% to $501.0 million.
- Consolidated revenue from oncology products of $271.5 million, up 65%.
- Net income of $37.7 million was achieved in 2024, with a cash balance of $836.1 million as of December 31, 2024, achieving financial self-reliance ahead of schedule.
- Agreed partial disposal of equity in SHPL3 joint venture for $608 million.
Pipeline progress and new technology platform
- Primary endpoint met in SACHI China Phase III interim analysis for savolitinib for EGFRm4 NSCLC5 with MET amplification, followed by swift NDA6 filing, acceptance and priority review granted by the NMPA7.
- Positive SAVANNAH global pivotal Phase II results for savolitinib in combination with TAGRISSO® for EGFRm NSCLC patients that progressed on TAGRISSO® treatment with MET overexpression or amplification, achieving high, clinically meaningful and durable response rate and shared with global regulatory authorities by AstraZeneca8.
- Positive FRUSICA-2 China Phase III results for fruquintinib with sintilimab in 2L9 RCC10.
- Presented ESLIM-01 China Phase III data at ASH11 and EHA12, highlighting strong, sustained, and long-term durable response rates of sovleplenib for ITP13 patients, with the NDA under review by the NMPA. Additional data were requested by CDE14 and subsequently submitted by HUTCHMED. Review of the supplementary data is currently under review by CDE.
- FRUSICA-1 Phase II results presented at ASCO15, leading to NMPA approval of a second indication of ELUNATE® (fruquintinib) for EMC16 with pMMR17
- First candidates from new ATTC18 platform, starting development of a new wave of drug candidates potentially more selective and tolerable than previous generations of antibody drug conjugates.
Dr Dan Eldar, Non-executive Chairman of HUTCHMED, said, “The successful commercialization of FRUZAQLA® outside of China by our partner Takeda and the resulting milestones achieved during the year were pivotal in helping HUTCHMED reach its profitability goals. I am proud that, at times of uncertainty in the global environment and in the capital markets, we have successfully established an independent ability to support our valuable discovery engine and development pipeline while mitigating operational risks. We expect to continue our global growth with further sales in the US and in other regions of the world, while continuing to develop our pipeline in new and promising directions. The long-term interests of our shareholders and benefits to patients around the world will always remain our top priorities.”
“At the end of 2024, we decided to dispose of our 45% equity interest in SHPL for $608 million, subject to closing conditions. I would like to take this opportunity to express my appreciation to the management team at SHPL for their contribution to its impressive growth over the last 20 years, which has delivered consistent benefits to consumers and shareholders alike. The commercial success and monetary contribution were important in supporting HUTCHMED’s novel drug R&D19, helping us to weather challenges in our industry as we developed innovative medicines for patients in need. As our innovative drugs business has become more self-reliant, we believe it is time for HUTCHMED to move on to our next phase of evolution, particularly as we focus on global clinical development of our ATTCs. The proceeds from the SHPL disposal, on top of the ongoing profits of our globally commercialized portfolio, enables us to expedite the roll-out of this differentiated platform, which will be key to our long-term value creation.”
Dr Weiguo Su, Chief Executive Officer and Chief Scientific Officer of HUTCHMED, said, “We’ve had a highly successful year, delivering against our strategy, in the clinic and commercially with our transformational medicines. This has culminated in HUTCHMED reaching profitability, which has been a key focus of ours. I’d like to thank and congratulate the team for this milestone, as we turn our attention to further growth and cultivating HUTCHMED’s next wave of medicines through our ATTC platform.”
“Our pioneering ATTC platform turns a new page in HUTCHMED’s innovative drug development story, establishing a new frontier in antibody-drug conjugates. This new portfolio of molecules is well placed to target a wide range of oncology indications with sizable market potential, including in first-line combinations. With the expertise and the financial strength to execute global clinical trials, we plan to move expeditiously into clinical development this year.”
“Our commercial medicines hit new milestones and expanded clinical development, reaching more patients in need around the world. Fruquintinib is now treating colorectal cancer patients in over a dozen countries, with more to come. FRUZAQLA® in-market sales exceeded $200 million within a year of launch, triggering the first sales milestone. In China, it was approved in second-line endometrial cancer, with average duration of treatment almost double that of fruquintinib’s first indication, and a third registrational study FRUSICA-2 has read out positively in kidney cancer.”
“For savolitinib, positive data from SACHI interim analysis in patients progressed on first line EGFR20 TKI21 treatment with MET amplification led us to file a NDA in China, which was accepted and granted priority review. We are hopeful that SAVANNAH/SAFFRON trials will support bringing this innovative medicine to patients globally. With recent full approval in both first-line and second-line MET exon 14 skipping alteration lung cancer, savolitinib remains one of the best-in-class medicines. A registration-intent study in MET-amplified gastric cancer is currently enrolling in China. We look forward to potentially expanding its indication as the first medicine for MET amplified EGFRm NSCLC and gastric cancer. Our marketed medicines will continue to support the revenue and earnings growth of HUTCHMED.”
“ESLIM-01 data for sovleplenib was presented at EHA and ASH, with durable response rate of 51.4% and overall response rate of 81.0%, significantly better than many different modalities of ITP medicines under development. These clinical results of sovleplenib again illustrate HUTCHMED’s R&D competency in selectivity, resulting in desirable efficacy and safety. We are working closely with the NMPA and look forward to bringing this innovative medicine to patients in need. ESLIM-02 registration Phase III in warm AIHA22 patients is enrolling and on-track to read out next year. A NDA is under review in China for tazemetostat for recurrent/refractory follicular lymphoma and approval is expected by mid-2025. We look forward to being able to add sovleplenib and tazemetostat to our commercial portfolio and their contributions to HUTCHMED’s continued growth.”
2024 FULL YEAR RESULTS & BUSINESS UPDATES
I. COMMERCIAL OPERATIONS
Oncology product in-market sales were up 134% (136% at CER23) to $501.0 million in 2024 (2023: $213.6m), leading to strong growth in oncology product consolidated revenue of 65% (67% at CER) to $271.5 million (2023: $164.2m).
- FRUZAQLA® (fruquintinib ex-China) in-market sales were $290.6 million in 2024 (2023: $15.1m) by Takeda, with strong performance reflecting rapid US patient uptake, as well as launches in over a dozen countries. Reaching $200.0 million sales triggered a $20 million milestone payment from Takeda.
- ELUNATE® (fruquintinib China) in-market sales increased 7% (9% at CER) to $115.0 million in 2024 (2023: $107.5m), maintaining its leading market share position in metastatic CRC24 and demonstrating resilience against rising pressure from competing products and their generics. New indication for EMC was approved in December 2024.
- SULANDA® (surufatinib) in-market sales increased 12% (14% at CER) to $49.0 million in 2024 (2023: $43.9m), as increasing brand awareness amongst doctors and improving NET25 diagnosis drives prescription growth and market share to 27% in 2024 (2023: 21%).
- ORPATHYS® (savolitinib) in-market sales approximated prior year (-2%, flat at CER) to $45.5 million in 2024 (2023: $46.1m), impacted by the launch and NRDL26 inclusion of several competing same-class MET TKIs for 2L METex1427 NSCLC. Results do not reflect full approval in 1L28 setting received in January 2025.
Total Oncology/Immunology consolidated revenue was $363.4 million in 2024 (2023: $528.6m), within guidance of $300 million to $400 million.
- Oncology product consolidated revenue (royalties, manufacturing revenue, promotion and marketing services revenue and commercial milestone) increased 65% (67% at CER) to $271.5 million (2023: $164.2m), driven by FRUZAQLA® and exceeding guidance of 30% to 50% growth.
- Takeda upfront, regulatory milestones and R&D services revenue were $67.0 million (2023: $345.9m), which included recognition of $48.1 million of the $450.0 million upfront and regulatory milestone payments achieved. This compared to recognition of $312.0 million in 2023.
- Other revenue was $24.9 million (2023: $18.5m), including milestone payment of $6.0 million from AstraZeneca following NDA acceptance in China for ORPATHYS® combined with TAGRISSO®.
$630.2 million total consolidated revenue (2023: $838.0m) including Other Ventures of $266.8 million (2023: $309.4m).
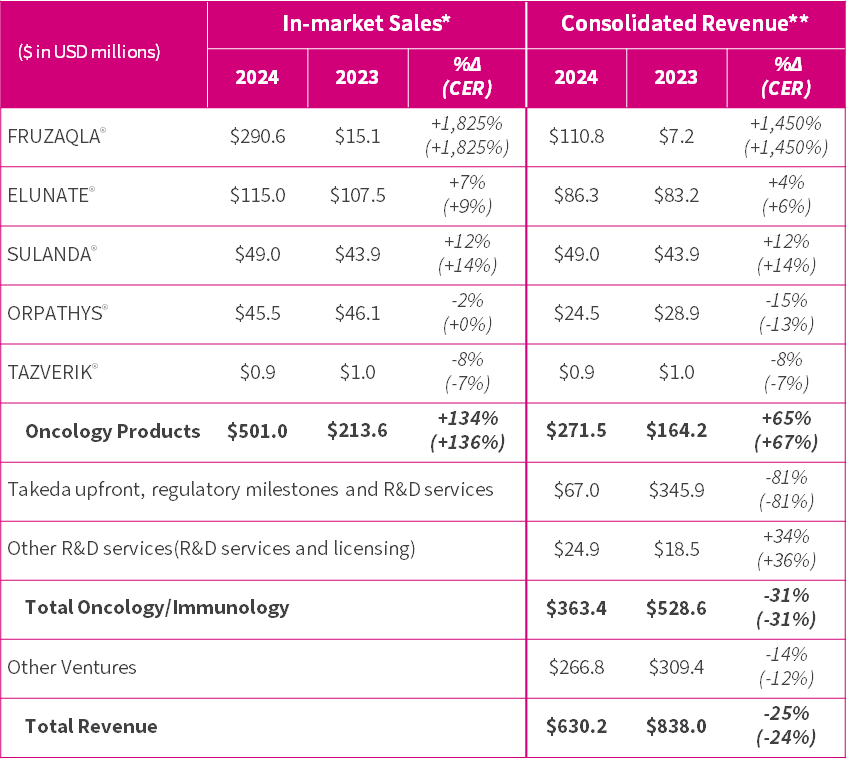
** = FRUZAQLA® represents manufacturing revenue, royalties and commercial milestone paid by Takeda; ELUNATE® represents manufacturing revenue, promotion and marketing services revenue and royalties paid by Lilly to HUTCHMED, and sales to other third parties invoiced by HUTCHMED; ORPATHYS® represents manufacturing revenue and royalties paid by AstraZeneca and sales to other third parties invoiced by HUTCHMED; SULANDA® and TAZVERIK® represent the Company’s sales of the products to third parties.
II. REGULATORY UPDATES
China
- Savolitinib NDA accepted by the NMPA with Priority Review status and Breakthrough Therapy designation for 2L EGFRm NSCLC patients with MET amplification, in combination with TAGRISSO® (osimertinib), in December 2024, triggering a milestone from AstraZeneca.
- Savolitinib sNDA30 approved by the NMPA for 1L and 2L (converted from conditional to full approval) METex14 NSCLC in January 2025.
- Fruquintinib sNDA approved by the NMPA, in combination with TYVYT® (sintilimab), for 2L EMC patients with pMMR status in December 2024.
- Fruquintinib approved in Hong Kong for 3L31 CRC under the new 1+ Mechanism in January 2024, and subsequently the first innovative oncology medicine enlisted with Full Subsidy under the Special Drug category in October 2024.
- Tazemetostat approved in Hong Kong for 3L R/R32 EZH2m33 follicular lymphoma in May 2024.
- Savolitinib approved in Hong Kong for METex14 NSCLC under the 1+ Mechanism in February 2025.
- Tazemetostat NDA accepted by the NMPA with Priority Review status for 3L R/R follicular lymphoma in July 2024.
- Fruquintinib sNDA voluntarily withdrawn for 2L gastric cancer, in combination with paclitaxel, in August 2024, in light of discussions with the NMPA and internal review of current data package.
Ex-China
- Fruquintinib approved in the EU for CRC in June 2024, followed by first European reimbursement in Spain in December 2024, triggering a $10.0 million milestone from Takeda.
- Fruquintinib approved in Japan for CRC in September 2024, followed by pricing approval and launch in November 2024, triggering a milestone from Takeda.
- Fruquintinib approved in Argentina and Switzerland in August 2024, in Canada (also with reimbursement) and the United Kingdom in September 2024, in Australia and Singapore in October 2024, in Israel and the United Arab Emirates in December 2024, and in South Korea in March 2025.
III. LATE-STAGE CLINICAL DEVELOPMENT ACTIVITIES
Savolitinib (ORPATHYS® in China), a highly selective oral inhibitor of MET
- Positive SAVANNAH global pivotal Phase II top-line results for 2L EGFRm NSCLC patients with MET amplification or overexpression, in combination with TAGRISSO® (osimertinib), achieving high, clinically meaningful and durable response rate (NCT03778229).
- Primary endpoint met in SACHI China Phase III interim analysis for 2L EGFRm NSCLC patients with MET amplification (NCT05015608).
- Presented Phase II small randomized controlled study results at AACR34 for 2L EGFRm NSCLC patients with high MET amplification, in combination with TAGRISSO® (osimertinib), showing ORR35 of 63% and median PFS36 of 8.2 months (NCT04606771).
- Continued enrolling SAFFRON global Phase III study for 2L EGFRm NSCLC patients with MET amplification or overexpression (NCT05261399) supporting SAVANNAH; and SANOVO China Phase III study for 1L EGFRm NSCLC patients with MET overexpression (NCT05009836).
Potential upcoming clinical and regulatory milestones for savolitinib:
- Presentation of SAVANNAH and SACHI data at upcoming scientific conferences.
- Complete SACHI NMPA NDA review in late 2025.
- Complete SAFFRON enrollment in the second half of 2025.
- Complete enrollment and potential NDA submission for gastric cancer with MET amplification in the second half of 2025.
Fruquintinib (ELUNATE® in China, FRUZAQLA® outside of China), a highly selective oral inhibitor of VEGFR37
- Presented FRUSICA-1 China pivotal Phase II results at ASCO, in combination with TYVYT®(sintilimab), for previously treated EMC with pMMR status, showing IRC38-assessed confirmed ORR of 35.6%, median PFS of 9.5 months and median OS39 of 21.3 months with a manageable safety profile (NCT03903705). This indication was approved by the NMPA in December 2024.
- Presented FRESCO-2 subgroup analyses for CRC patients at ASCO, biomarker analysis at AACR and quality-of-life analysis at ASCO GI40, showing meaningful quality-adjusted survival benefit, efficacy regardless of prior therapy or sequence as well as CEA41 potentially a predictor of efficacy (NCT04322539).
- Published FRUTIGA China Phase III results in Nature Medicine for 2L gastric cancer, in combination with paclitaxel, and presentations at ASCO, showing statistically significant improvements in ORR and PFS, as well as OS benefits in sub-group without taking subsequent antitumor therapy (NCT03223376).
- Positive result of FRUSICA-2 China Phase III in 2L RCC in March 2025 (NCT05522231).
Sovleplenib (HMPL-523), an investigative and highly selective oral inhibitor of Syk42
- Published ESLIM-01 China Phase III results for adult patients with primary ITP in China in The Lancet Haematology concurrently with presentations at EHA, showing durable response rate of 48.4%, tolerable safety profile and improved quality of life regardless of prior lines of therapies (NCT05029635).
- Presented ESLIM-01 China Phase III long-term results at ASH, showing durable response rate of 51.4% and long-term durable response rate of 59.8% as well as consistent safety profile.
- Published China Phase II results in warm AIHA in China at EHA and in The Lancet Haematology in 2025, demonstrating overall response rate of 66.7% and a favorable safety profile (NCT05535933).
- Initiated ESLIM-02 China Phase III stage in warm AIHA (NCT05535933).
Potential upcoming clinical milestones for sovleplenib:
- Complete ESLIM-01 NMPA NDA review around end 2025 (NCT05029635).
- Complete enrollment of ESLIM-02 Phase III in the second half of 2025 (NCT05535933).
Surufatinib (SULANDA® in China), an oral inhibitor of VEGFR, FGFR43 and CSF-1R44
- Completed enrollment of Phase II part of a China Phase II/III trial for 1L metastatic PDAC45 patients, in combination with AiRuiKa® (camrelizumab), nab-paclitaxel and gemcitabine (NCT06361888). This study was informed in part by an investigator-initiated trial presented at ASCO GI 2024 of a similar combination.
Potential upcoming clinical milestone for surufatinib:
- Data readout of the PDAC Phase II trial in late 2025.
Tazemetostat (TAZVERIK® in Hainan, Macau and Hong Kong), a first-in-class, oral inhibitor of EZH2
- Positive bridging study in 3L follicular lymphoma leading to NDA submission with Priority Review status (NCT05467943).
- Continued enrolling SYMPHONY-1 Phase III China portion of the global study, in combination with lenalidomide and rituximab, in follicular lymphoma patients (NCT04224493).
Potential upcoming clinical milestone for tazemetostat:
- Complete NDA review in China in mid 2025.
Fanregratinib (HMPL-453), a novel, highly selective and potent inhibitor targeting FGFR 1, 2 and 3
- Completed enrollment of registrational China pivotal Phase II for IHCC46 with FGFR2 fusion / rearrangement in March 2025 (NCT04353375).
Ranosidenib (HMPL-306), an investigative and highly selective oral dual-inhibitor of IDH1 and IDH247 enzymes
- Presented and published results from China and US/European Phase I studies at EHA and the journal Med for R/R IDH1/2m48 AML49 patients (NCT04272957, NCT04764474).
- Initiated RAPHAEL China Phase III trial for 2L R/R IDH1/2m AML (NCT06387069).
Other early-stage investigational drug candidates
- Presented pre–clinical and Phase I results at AACR, ASCO and EHA for ERK1/250 inhibitor HMPL-295, third-generation BTK51 inhibitor HMPL-760, Menin inhibitor HMPL-506, and anti-CD38 HMPL-A067.
- Initiated Phase I trial for HMPL-506 in hematological malignancies in China (NCT06387082).
IV. ANTIBODY-TARGETED THERAPY CONJUGATE (ATTC) PLATFORM
New in-house created platform with multiple potential IND52 candidates
Our ATTC next-generation technology platform leverages over 20 years of expertise in targeted therapies with small molecules inhibitors. ATTC drug candidates enrich the next wave of clinical development with potential key advantages over traditional antibody-drug conjugates and/or small molecule medicines:
- Better efficacy through synergistic antibody-small molecule targeted therapy combinations that will target specific mutations; overcome drug resistance and potentially support combinations with other targeted therapies, chemotherapy and immunotherapy, in early-line patient settings.
- Improved safety and prolonged treatment given lower off-tumor or off-target toxicity than small molecules, less myelosuppression and better quality of life than cytotoxin-based conjugates.
- Attractive pharmacokinetics tackles difficult drug targets, enabled by antibody-guided delivery to target sites which will improve bioavailability and reduce drug-drug interactions when compared to oral small molecules inhibitors.
V. COLLABORATION UPDATES
Further progress by Inmagene53 with two candidates discovered by HUTCHMED
- HUTCHMED received 7.5% shareholding interest in Inmagene following the latter’s exercise of an option to exclusively develop, manufacture and commercialize IMG-007, a nondepleting anti-OX40 antibody, and IMG-004, a reversible, non-covalent, highly selective oral BTK inhibitor.
- Inmagene and Ikena Oncology, Inc. (Nasdaq: IKNA) agreed to merge, which is expected to close in mid-2025, subject to closing conditions. HUTCHMED will have an interest in the merged company.
- Inmagene announced positive results of a Phase IIa trial with IMG-007 for atopic dermatitis, showing Week 16 mean change in EASI54 of 77% and EASI-75 response of 54% (NCT05984784). A Phase IIb dose-finding study with a subcutaneous formulation in moderate-to-severe atopic dermatitis is planned.
- Inmagene enrolled a Phase IIa trial with IMG-007 for alopecia areata (NCT06060977), and announced results of a Phase I study with IMG-004, indicating once daily dosing potential (NCT05349097).
VI. OTHER VENTURES
- Other Ventures consolidated revenue is predominantly from the prescription drug distribution business55 in China. It decreased by 14% (12% at CER) to $266.8 million (2023: $309.4m) primarily due to lower COVID-related prescription drug distribution sales in 2024.
- Share of equity in earnings of SHPL, a non-consolidated joint venture, slightly decreased by 2% (increased 1% at CER) to $46.5 million (2023: $47.4m) mainly due to increased clinical trial investment for new products.
- Consolidated net income attributable to HUTCHMED from Other Ventures decreased by 5% (2% at CER) to $47.7 million (2023: $50.3m), due to disposal of consumer products business in December 2023, lower COVID-related prescription drug distribution sales and fluctuation in net income contributed from SHPL.
SHPL Disposal: HUTCHMED entered into share purchase agreements to divest its 45.0% equity interest in SHPL for approximately $608 million in cash, retaining a 5.0% equity interest. It is estimated that HUTCHMED will record a pre-tax gain of approximately $477 million.
VII. SUSTAINABILITY
HUTCHMED is committed to progressively embedding sustainability into all aspects of its operations and creating long-term value for its stakeholders. Continued progress was made in 2024 including:
- Sustainability goals and targets: satisfactory progress made in 11 short- to long-term goals and targets; sustainability performance continued to be incorporated into management’s performance-based remuneration. To prepare for new targets setting, sustainability-related efforts were continually assessed and a target achievement roadmap focused on HUTCHMED’s five sustainability pillars is being developed.
- Enhanced climate actions: based on the 2022 climate risk assessment, HUTCHMED conducted another comprehensive assessment on the potential financial impacts of climate risks and opportunities for HUTCHMED with costs estimated under low-, mid-, and high-emission scenarios. This also prepares it for the latest climate-related disclosure requirements of the HKEX56 and other international disclosure standards.
- Biodiversity assessment: a biodiversity assessment was conducted to understand HUTCHMED’s dependency and impact on nature. Based on the results of the assessment, a Biodiversity Policy was prepared and approved by the Board for public disclosure.
- Supplier ESG57 assessment: this was conducted to understand the sustainability maturity of the supplier base and pave the way for a tailored supplier engagement program in 2025.
- Improvement on ESG ratings: MSCI ESG upgraded the rating of HUTCHMED from BBB to A. ISS ESG upgraded the rating of HUTCHMED from C to C+, which is classified as Prime. Its S&P Global ESG score continued to rise from 48 to 53, placing HUTCHMED in the 90th percentile of the industry. Additionally, HUTCHMED achieved an A- rating and a top quartile score in the Hang Seng Corporate Sustainability Index Series rating, particularly in the areas of environment and governance.
In recognition of its marked improvement in sustainability efforts within the pharmaceutical industry, HUTCHMED was honored with multiple ESG awards in 2024. These efforts will continue to guide HUTCHMED towards a more sustainable future. The 2024 Sustainability Report will be published alongside the 2024 Annual Report in April 2025 and will include further information on sustainability initiatives and performance.
Financial Highlights
Foreign exchange impact: The RMB depreciated against the US dollar by approximately 3% during 2024 on average, which has impacted consolidated financial results as highlighted below.
Revenue for the year ended December 31, 2024 was $630.2 million compared to $838.0 million in 2023.
- Oncology/Immunology consolidated revenue amounted to $363.4 million (2023: $528.6m):
- FRUZAQLA® revenue was $110.8 million, reflecting its successful launch since November 2023 comprising royalties, manufacturing revenue and commercial milestone.
- ELUNATE® revenue increased 4% (6% at CER) to $86.3 million (2023: $83.2m) in its sixth year since launch, comprising of manufacturing revenue, promotion and marketing services revenue and royalties, maintaining its leading market share position while weathering greater market competition.
- SULANDA® revenue increased 12% (14% at CER) to $49.0 million (2023: $43.9m) due to continued sales growth after NRDL renewal as brand awareness amongst doctors continues to increase, leading to greater NET patient access and market share.
- ORPATHYS® revenue decreased 15% (13% at CER) to $24.5 million (2023: $28.9m), due to phasing of manufacturing revenue of $10.9 million (2023: $15.1m), and royalties of $13.6 million (2023: $13.8m).
- TAZVERIK® revenue was $0.9 million (2023: $1.0m) mainly from sales in Hainan and Hong Kong.
- Takeda upfront, regulatory milestones and R&D services revenue decreased to $67.0 million (2023: $345.9m, of which $280.0m was the recognized portion of the $400.0 million upfront cash payment received from Takeda in April 2023).
- Other revenue of $24.9 million (2023: $18.5m), primarily related to milestone payment of $6.0 million from AstraZeneca and fees from AstraZeneca and Lilly for development and regulatory activities.
- Other Ventures consolidated revenue decreased 14% (12% at CER) to $266.8 million (2023: $309.4m), primarily as a result of lower COVID-related prescription drug distribution sales in 2024. This excluded non-consolidated revenue at SHPL of $393.5 million (2023: $385.5m).
Net Expenses for 2024 were $592.5 million compared to $737.2 million in 2023, reflecting strong efforts on cost control.
- Cost of Revenue decreased by 9% to $348.9 million (2023: $384.4m), which was mainly due to lower revenue from Other Ventures. Cost of revenue as a percentage of oncology product revenue improved (from 56% in 2023 to 34% in 2024) due to favorable product mix and economies of scale.
- R&D Expenses reduced 30% to $212.1 million (2023: $302.0m), mainly due to restructuring of teams outside of China, with clinical and regulatory expenses in the US and Europe decreasing to $34.5 million (2023: $106.9m). China investment was $177.6 million (2023: $195.1m) which reflects both a decrease in cost for completed studies with NDAs under review and an ongoing commitment to key assets with global potential in our internal pipeline, including the development of the next-generation ATTC platform.
- S&A58 Expenses were $112.9 million (2023: $133.2m), which decreased primarily due to tighter controls over administrative spending $64.3 million (2023: $79.8m) and lower selling expenses $48.6 million (2023: $53.4m) as we realized efficiencies from a salesforce already scaled to support revenue growth.
- Other Items mainly comprised of equity in earnings of SHPL, interest income and expense, FX and taxes, generated net income of $81.4 million (2023: $82.4m).
Net Income attributable to HUTCHMED for 2024 was $37.7 million compared to $100.8 million in 2023.
- The net income attributable to HUTCHMED in 2024 was $0.04 per ordinary share / $0.22 per ADS59, (2023: $0.12 per ordinary share / $0.59 per ADS).
Cash, Cash Equivalents and Short-Term Investments were $836.1 million as of December 31, 2024 compared to $886.3 million as of December 31, 2023.
- Adjusted Group (non-GAAP60) net cash flows excluding financing activities in 2024 were -$19.5 million mainly due to net income attributable to HUTCHMED of $37.7 million offset by changes in working capital of $62.2 million from partner milestones achieved and receivable at the end of 2024 and ongoing recognition of Takeda deferred revenue (2023: $206.7m due to the receipt of $435 million in upfront and milestone payments from Takeda).
- Net cash used in financing activities in 2024 totaled $30.7 million mainly due to purchases for equity awards of $36.1 million (2023: net cash generated from financing activities of $48.7m mainly due to drawdowns of bank borrowings).
FINANCIAL GUIDANCE
HUTCHMED provides full year 2025 guidance for Oncology/Immunology consolidated revenue of $350 million to $450 million. HUTCHMED’s work in 2025 and beyond will be supported by its strong balance sheet. The Company will continue to be financially self-reliant while supporting investments to bring innovative medicines to patients globally.
Shareholders and investors should note that:
- The Company does not provide any guarantee that the statements contained in the financial guidance will materialize or that the financial results contained therein will be achieved or are likely to be achieved; and
- The Company has in the past revised its financial guidance and reference should be made to any announcements published by it regarding any updates to the financial guidance after the date of publication of this announcement.
———
Use of Non-GAAP Financial Measures and Reconciliation – References in this announcement to adjusted Group net cash flows excluding financing activities and financial measures reported at CER are based on non-GAAP financial measures. Please see the “Use of Non-GAAP Financial Measures and Reconciliation” for further information relevant to the interpretation of these financial measures and reconciliations of these financial measures to the most comparable GAAP measures, respectively.
———
FINANCIAL STATEMENTS
HUTCHMED will today file with the US Securities and Exchange Commission its Annual Report on Form 20-F.
FINANCIAL SUMMARY
Condensed Consolidated Balance Sheets Data
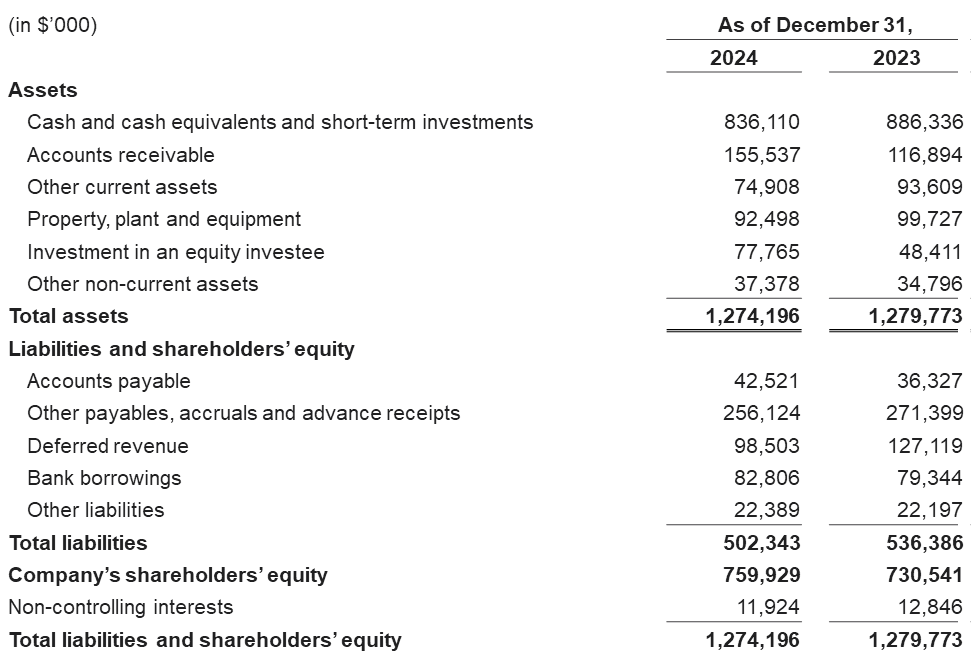
Condensed Consolidated Statements of Operations Data
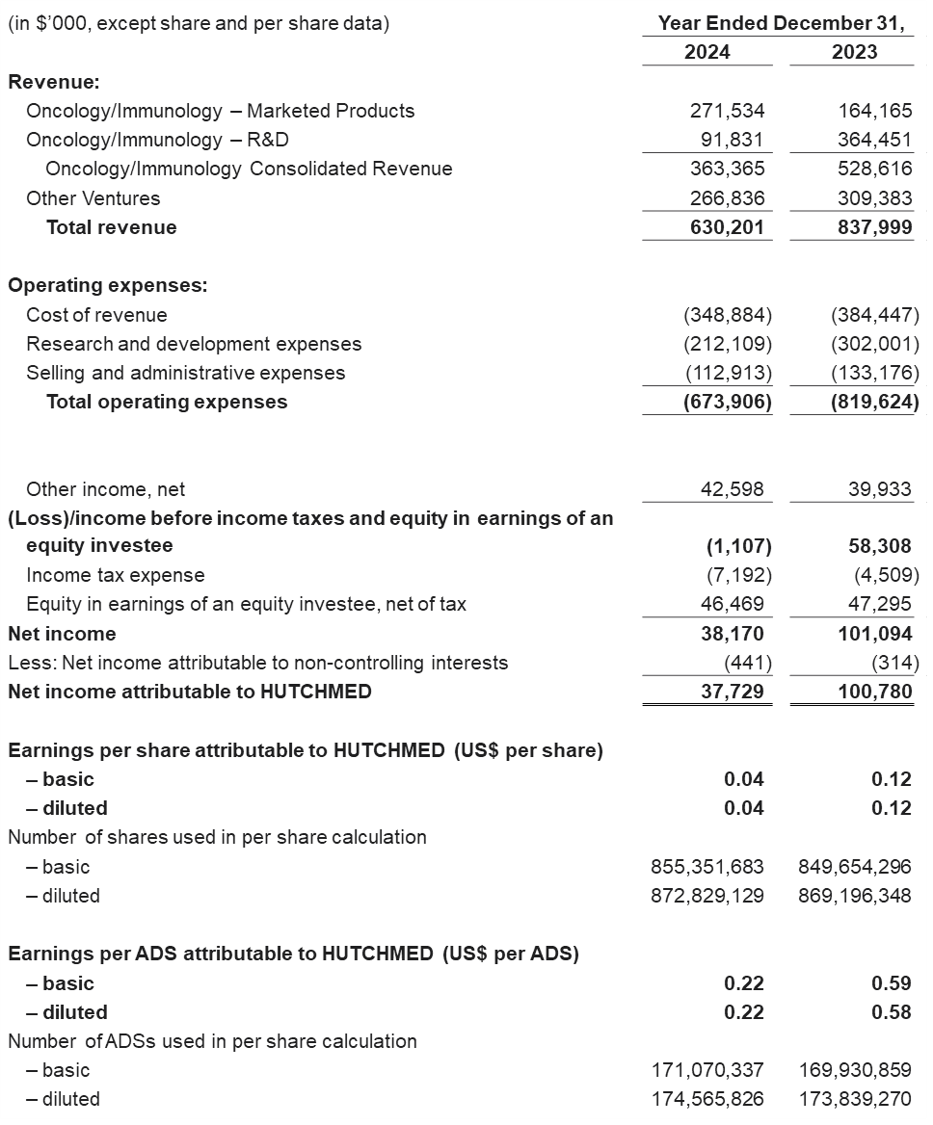
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, and the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Contacts
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
HSBC |
Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
Cavendish |
Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
Unless the context requires otherwise, references in this announcement to the “Group,” the “Company,” “HUTCHMED,” “HUTCHMED Group,” “we,” “us,” and “our,” mean HUTCHMED (China) Limited and its subsidiaries unless otherwise stated or indicated by context.
Past Performance and Forward-Looking Statements
The performance and results of operations of the Group contained within this announcement are historical in nature, and past performance is no guarantee of future results of the Group. This announcement contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements can be identified by words like “will,” “expects,” “anticipates,” “future,” “intends,” “plans,” “believes,” “estimates,” “pipeline,” “could,” “potential,” “first-in-class,” “best-in-class,” “designed to,” “objective,” “guidance,” “pursue,” or similar terms, or by express or implied discussions regarding potential drug candidates, potential indications for drug candidates or by discussions of strategy, plans, expectations or intentions. You should not place undue reliance on these statements. Such forward-looking statements are based on the current beliefs and expectations of management regarding future events, and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. There can be no guarantee that any of our drug candidates will be approved for sale in any market, that any approvals which have been obtained will continue to remain valid and effective in the future, or that the sales of products marketed or otherwise commercialized by HUTCHMED and/or its collaboration partners (collectively, “HUTCHMED’s Products”) will achieve any particular revenue or net income levels. In particular, management’s expectations could be affected by, among other things: unexpected regulatory actions or delays or government regulation generally; the uncertainties inherent in research and development, including the inability to meet our key study assumptions regarding enrollment rates, timing and availability of subjects meeting a study’s inclusion and exclusion criteria and funding requirements, changes to clinical protocols, unexpected adverse events or safety, quality or manufacturing issues; the delay or inability of a drug candidate to meet the primary or secondary endpoint of a study; the delay or inability of a drug candidate to obtain regulatory approval in different jurisdictions or the utilization, market acceptance and commercial success of HUTCHMED’s Products after obtaining regulatory approval; discovery, development and/or commercialization of competing products and drug candidates that may be superior to, or more cost effective than, HUTCHMED’s Products and drug candidates; the impact of studies (whether conducted by HUTCHMED or others and whether mandated or voluntary) or recommendations and guidelines from governmental authorities and other third parties on the commercial success of HUTCHMED’s Products and drug candidates in development; the ability of HUTCHMED to manufacture and manage supply chains, including various third party services, for multiple products and drug candidates; the availability and extent of reimbursement of HUTCHMED’s Products from third-party payers, including private payer healthcare and insurance programs and government insurance programs; the costs of developing, producing and selling HUTCHMED’s Products; the ability to obtain additional funding when needed; the ability to obtain and maintain protection of intellectual property for HUTCHMED’s Products and drug candidates; the ability of HUTCHMED to meet any of its financial projections or guidance and changes to the assumptions underlying those projections or guidance; the successful disposition of its non-core business; global trends toward health care cost containment, including ongoing pricing pressures; uncertainties regarding actual or potential legal proceedings, including, among others, actual or potential product liability litigation, litigation and investigations regarding sales and marketing practices, intellectual property disputes, and government investigations generally; and general economic and industry conditions, including uncertainties regarding the effects of the persistently weak economic and financial environment in many countries, uncertainties regarding future global exchange rates, uncertainties in global interest rates, and geopolitical relations, sanctions and tariffs. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, on AIM and on HKEX. HUTCHMED is providing the information in this announcement as of this date and does not undertake any obligation to update any forward-looking statements as a result of new information, future events or otherwise.
In addition, this announcement contains statistical data and estimates that HUTCHMED obtained from industry publications and reports generated by third-party market research firms. Although HUTCHMED believes that the publications, reports and surveys are reliable, HUTCHMED has not independently verified the data and cannot guarantee the accuracy or completeness of such data. You are cautioned not to give undue weight to this data. Such data involves risks and uncertainties and are subject to change based on various factors, including those discussed above.
Inside Information
This announcement contains inside information for the purposes of Article 7 of Regulation (EU) No 596/2014 (as it forms part of retained EU law as defined in the European Union (Withdrawal) Act 2018).
Medical Information
This announcement contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
Ends
1 In-market sales = total sales to third parties provided by Eli Lilly (ELUNATE®), Takeda (FRUZAQLA®), AstraZeneca (ORPATHYS®) and HUTCHMED (ELUNATE®, SULANDA®, ORPATHYS® and TAZVERIK®).
2 Takeda = Takeda Pharmaceuticals International AG, a subsidiary of Takeda Pharmaceutical Company Limited.
3 SHPL = Shanghai Hutchison Pharmaceuticals Limited.
4 EGFRm = Epidermal growth factor receptor mutated.
5 NSCLC = Non-small cell lung cancer.
6 NDA = New Drug Application.
7 NMPA = China National Medical Products Administration.
8 AstraZeneca = AstraZeneca AB, a subsidiary of AstraZeneca plc.
9 2L = Second-line.
10 RCC = Renal cell carcinoma.
11 ASH = American Society of Hematology.
12 EHA = European Hematology Association.
13 ITP = immune thrombocytopenia purpura.
14 CDE = Centre for Drug Evaluation.
15 ASCO = American Society of Clinical Oncology.
16 EMC = Endometrial cancer.
17 pMMR = Proficient mismatch repair.
18 ATTC = antibody-targeted therapy conjugates.
19 R&D = Research and development.
20 EGFR = Epidermal growth factor receptor.
21 TKI = Tyrosine kinase inhibitor.
22 AIHA = Autoimmune hemolytic anemia.
23 CER = Constant exchange rate. We also report changes in performance at CER which is a non-GAAP measure. Please refer to “Use of Non-GAAP Financial Measures and Reconciliation” for further information relevant to the interpretation of these financial measures and reconciliations of these financial measures to the most comparable GAAP measures.
24 CRC = Colorectal cancer.
25 NET = Neuroendocrine tumor.
26 NRDL = China National Reimbursement Drug List.
27 METex14 = MET exon 14 skipping alteration.
28 1L = First-line.
29 Lilly = Eli Lilly and Company.
30 sNDA = Supplemental New Drug Application.
31 3L = Third-line.
32 R/R = Relapsed and/or refractory.
33 EZH2m = Enhancer of zeste homolog 2 mutated.
34 AACR = American Association for Cancer Research.
35 ORR = Objective response rate.
36 PFS = Progression free survival.
37 VEGFR = Vascular endothelial growth factor receptor.
38 IRC = Independent review committee.
39 OS = Overall survival.
40 ASCO GI = ASCO Gastrointestinal Cancers Symposium.
41 CEA = Carcinoembryonic antigen.
42 Syk = Spleen tyrosine kinase.
43 FGFR = Fibroblast growth factor receptor.
44 CSF-1R = Colony-stimulating factor 1 receptor.
45 PDAC = Pancreatic ductal adenocarcinoma.
46 IHCC = Intrahepatic cholangiocarcinoma.
47 IDH1 and IDH2 = Isocitrate dehydrogenase-1 and isocitrate dehydrogenase-2.
48 IDH1/2m = Isocitrate dehydrogenase-1 OR isocitrate dehydrogenase-2 mutated.
49 AML = Acute myeloid leukemia.
50 ERK = Extracellular signal-regulated kinase.
51 BTK = Bruton’s tyrosine kinase.
52 IND = Investigational new drug application.
53 Inmagene = Inmagene Biopharmaceuticals.
54 EASI = Eczema area and severity index.
55 Distribution business = Shanghai Hutchison Whampoa Pharmaceuticals Sales Limited, formerly Hutchison Whampoa Sinopharm Pharmaceuticals (Shanghai) Company Limited.
56 HKEX = The Main Board of The Stock Exchange of Hong Kong Limited.
57 ESG = Environmental, Social and Governance.
58 S&A = Selling and administrative expenses.
59 ADS = American depositary share.
60 GAAP = Generally Accepted Accounting Principles.
| Announcement release: Mar 19, 2025 (Wed) |
| 7am EDT / 11am GMT / 7pm HKT |
| >> View Announcement<< |
| Presentation webcast & call |
| ⯈ English Session: Mar 19, 2025 (Wed) |
| 8am EDT / 12pm GMT / 8pm HKT |
| Listen to Webcast Replay (English Session) |
| ⯈ Chinese (Putonghua) Session: Mar 20, 2025 (Thu) |
| 12:30am GMT / 8:30am HKT / (8:30pm EDT of Mar 19) |
| Listen to Webcast Replay (Chinese Session) |
Hong Kong, Shanghai & Florham Park, NJ — Wednesday, March 19, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) and Innovent Biologics, Inc. (“Innovent”) (HKEX: 01801), today jointly announce that the FRUSICA-2 Phase II/III clinical trial evaluating fruquintinib in combination with sintilimab as second-line treatment for locally advanced or metastatic renal cell carcinoma (“RCC”) in China has met its primary endpoint of progression free survival (“PFS”) per RECIST 1.1 as assessed by blinded independent central review (BICR).
The combination of fruquintinib and sintilimab received conditional approval from the China National Medical Products Administration (“NMPA”) for the treatment of patients with advanced endometrial cancer with Mismatch Repair proficient (pMMR) tumors that have failed prior systemic therapy and are not candidates for curative surgery or radiation, based on data from the FRUSICA-1 study (NCT03903705).
The FRUSICA-2 study is a randomized, open-label, active-controlled study to evaluate the efficacy and safety of fruquintinib in combination with sintilimab versus axitinib or everolimus monotherapy for the second-line treatment of advanced RCC (NCT05522231). In addition to the primary endpoint PFS, the combination also demonstrated improvements in secondary endpoints including objective response rate (“ORR”) and duration of response (“DoR”). Full results will be submitted for presentation at an upcoming scientific conference.
Prof Dingwei Ye of Fudan University Shanghai Cancer Center and the co-leading Principal Investigator of the FRUSICA-2 study, said, “The rapid advancements in targeted therapies, immunotherapies, and their combination regimens have led to a significant evolution in the treatment landscape for advanced renal cell carcinoma. Targeted therapy remains an indispensable and crucial component in systemic treatment of advanced RCC in China. Optimizing the selection of targeted therapy, either as monotherapy or in combination with immunotherapy, for individual patients is a key focus of clinical interest. The results from the FRUSICA‑2 study underscore the potential of the fruquintinib and sintilimab combination to address the pressing medical needs of patients with this challenging disease.”
Prof Zhisong He of Peking University First Hospital and the co-leading Principal Investigator of the FRUSICA-2 study, said, “The positive results from this Phase III study of the fruquintinib and sintilimab combination represent a significant advancement in the treatment of advanced renal cell carcinoma. We are optimistic about the clinical implications of the findings as we strive to provide more effective treatment options for patients who may not have had adequate responses to previous therapies.”
“The encouraging results from our study provide clear evidence for the combination of fruquintinib and sintilimab as a viable new treatment option for advanced renal cell carcinoma patients who have progressed on previous therapy. This not only reaffirms our commitment to advancing cancer therapies but also represents an important step forward in addressing unmet medical needs within this patient population,” said Dr Michael Shi, Head of R&D and Chief Medical Officer of HUTCHMED. “I extend my heartfelt gratitude to the patients and investigators who participated in this research; their contributions have been vital to our success. We look forward to sharing detailed findings with regulatory authorities and progressing toward NDA filings in the coming months.”
“We are encouraged by the positive results in the FRUSICA-2 clinical trial. These outcomes not only underscore the great potential of the combination therapy of sintilimab and fruquintinib but also bring new hope to previously-treated patients with advanced renal cell carcinoma. We look forward to working closely with HUTCHMED to jointly advance the registrational communication of this innovative combo therapy and make it available to patients as soon as possible,” said Dr Hui Zhou, Senior Vice President of Innovent.
About Kidney Cancer and RCC
It is estimated that approximately 435,000 new patients were diagnosed with kidney cancer worldwide in 2022.[1] In China, an estimated 74,000 new patients were diagnosed with kidney cancer in 2022.[2] Approximately 90% of kidney tumors are RCC.
About Fruquintinib
Fruquintinib is a selective oral inhibitor of all three vascular endothelial growth factor (“VEGF”) receptors (VEGFR-1, -2 and -3). VEGFR inhibitors play a pivotal role in inhibiting tumor angiogenesis. Fruquintinib was designed to have enhanced selectivity that limits off-target kinase activity, allowing for drug exposure that achieves sustained target inhibition and flexibility for potential use as part of a combination therapy.[3]
About Fruquintinib Approvals
Fruquintinib is co-developed and co-marketed in China by HUTCHMED and Eli Lilly and Company under the brand name ELUNATE®. It is approved for the treatment of patients with metastatic colorectal cancer who have previously received fluoropyrimidine, oxaliplatin and irinotecan-based chemotherapy, and those who have previously received or are not suitable for receiving anti-VEGF therapy or anti-epidermal growth factor receptor (EGFR) therapy (RAS wild-type) in China. It was included in the China National Reimbursement Drug List (NRDL) in January 2020. Since its launch in China, over 100,000 patients with colorectal cancer have been treated with fruquintinib.
The combination of ELUNATE® (fruquintinib) and TYVYT® (sintilimab injection) has conditional approval in China for the treatment of patients with advanced endometrial cancer with Mismatch Repair proficient (pMMR) tumors that have failed prior systemic therapy and are not candidates for curative surgery or radiation.
Takeda holds the exclusive worldwide license to further develop, commercialize, and manufacture fruquintinib outside mainland China, Hong Kong and Macau, marketing it under the brand name FRUZAQLA®. Fruquintinib received approval for the treatment of previously treated metastatic colorectal cancer in the US in November 2023, in the EU in June 2024, in Switzerland and Argentina in August 2024, in Canada, Japan and the United Kingdom in September 2024, in Australia and Singapore in October 2024 and in Israel and the United Arab Emirates in December 2024. Regulatory applications are progressing in many other jurisdictions.
The global regulatory submissions are based on data from two large, randomized, controlled Phase III trials in colorectal cancer, the global, multi-regional FRESCO-2 trial and the FRESCO trial conducted in China, showing consistent benefit among a total of 734 metastatic colorectal cancer patients treated with fruquintinib. Safety profiles were consistent across trials. Results from the FRESCO-2 trial were published in The Lancet in June 2023,[4] while results from the FRESCO trial were published in The Journal of the American Medical Association, JAMA.[5]
The safety and efficacy of fruquintinib for the following investigational uses have not been established and there is no guarantee that it will receive health authority approval or become commercially available in any country for the uses being investigated:
About Fruquintinib for Second-line Treatment of RCC
The U.S. Food and Drug Administration (FDA) has approved five immune-oncology combination therapies for the first-line treatment of advanced RCC. However, only one immune-oncology combination therapy has been approved in China for advanced RCC patients classified as having intermediate or poor risk by the International mRCC Database Consortium (IMDC). Single-agent targeted therapy continues to be one of the primary choices for first-line treatment of advanced RCC in China. Notably, advanced RCC patients who have experienced failure with single-agent targeted therapy previously still indicate an unmet medical need.
Results from a proof-of-concept Phase Ib/II study of fruquintinib plus sintilimab were published in Targeted Oncology in January 2025. The combination demonstrated promising efficacy and a tolerable safety profile in this setting. At the data cutoff of October 9, 2024, all 20 enrolled previously treated patients were evaluable for efficacy, with a median follow-up duration of 45.7 months. The confirmed ORR was 60.0% and DCR was 85.0%. Median DoR was 13.9 months and median PFS was 15.9 months. Overall survival (“OS”) was not reached, and the 36-month OS rate was 58.3%.[6]
About Sintilimab
Sintilimab, marketed as TYVYT® (sintilimab injection) in China, is a PD-1 immunoglobulin G4 monoclonal antibody co-developed by Innovent and Eli Lilly and Company. Sintilimab is a type of immunoglobulin G4 monoclonal antibody, which binds to PD-1 molecules on the surface of T-cells, blocks the PD-1 / PD-Ligand 1 (PD-L1) pathway, and reactivates T-cells to kill cancer cells. [7]
In China, sintilimab has been approved and included in the updated NRDL for seven indications. The updated NRDL reimbursement scope for TYVYT® (sintilimab injection) includes:
- For the treatment of relapsed or refractory classic Hodgkin’s lymphoma after two lines or later of systemic chemotherapy;
- For the first-line treatment of unresectable locally advanced or metastatic non-squamous non-small cell lung cancer lacking EGFR or ALK driver gene mutations;
- For the treatment of patients with EGFR-mutated locally advanced or metastatic non-squamous non-small cell lung cancer who progressed after EGFR-TKI therapy;
- For the first-line treatment of unresectable locally advanced or metastatic squamous non-small cell lung cancer;
- For the first-line treatment of unresectable or metastatic hepatocellular carcinoma with no prior systematic treatment;
- For the first-line treatment of unresectable locally advanced, recurrent or metastatic esophageal squamous cell carcinoma;
- For the first-line treatment of unresectable locally advanced, recurrent or metastatic gastric or gastroesophageal junction adenocarcinoma.
Furthermore, sintilimab’s eighth indication, in combination with fruquintinib for the treatment of patients with advanced endometrial cancer with pMMR tumors that have failed prior systemic therapy and are not candidates for curative surgery or radiation, was conditional approved by the NMPA in December 2024. And the NDA for sintilimab in combination with ipilimumab as neoadjuvant treatment for resectable MSI-H/dMMR colon cancer is under the NMPA review and has been granted Priority Review designation.
In addition, three clinical studies of sintilimab have met their primary endpoints:
- Phase 2 study of sintilimab monotherapy as second-line treatment of esophageal squamous cell carcinoma;
- Phase 3 study of sintilimab monotherapy as second-line treatment for squamous non-small cell lung cancer with disease progression following platinum-based chemotherapy;
- Phase 2/3 study of sintilimab in combination with fruquintinib versus axitinib or everolimus monotherapy for the second-line treatment of advanced RCC.
Statement: Innovent does not recommend the use of any unapproved drug(s)/indication(s).
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery, global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception, HUTCHMED has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit www.hutch-med.com or follow us on LinkedIn.
About Innovent
Innovent is a leading biopharmaceutical company founded in 2011 with the mission to empower patients worldwide with affordable, high-quality biopharmaceuticals. The company discovers, develops, manufactures and commercializes innovative medicines that target some of the most intractable diseases. Its pioneering therapies treat cancer, cardiovascular and metabolic, autoimmune and eye diseases. Innovent has launched 15 products in the market. It has 3 new drug applications under regulatory review, 3 assets in Phase III or pivotal clinical trials and 16 more molecules in early clinical stage. Innovent partners with over 30 global healthcare companies, including Lilly, Sanofi, Incyte, Adimab, LG Chem and MD Anderson Cancer Center.
Guided by the motto, “Start with Integrity, Succeed through Action,” Innovent maintains the highest standard of industry practices and works collaboratively to advance the biopharmaceutical industry so that first-rate pharmaceutical drugs can become widely accessible. For more information, visit www.innoventbio.com, or follow Innovent on Facebook and LinkedIn.
Statement:
(1)Innovent does not recommend the use of any unapproved drug (s)/indication(s).
(2)Ramucirumab (Cyramza®) and Selpercatinib (Retsevmo®) and Pirtobrutinib (Jaypirca®) were developed by Eli Lilly and Company.
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the therapeutic potential of the fruquintinib and sintilimab combination for the treatment of patients with RCC and the further clinical development of the fruquintinib and sintilimab combination in this and other indications. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding the timing and outcome of clinical studies and the sufficiency of clinical data to support NDA approval of the fruquintinib and sintilimab combination for the treatment of patients with RCC or other indications in China or other jurisdictions, its potential to gain approvals from regulatory authorities on an expedited basis or at all, the safety profile of the fruquintinib and sintilimab combination, HUTCHMED’s ability to fund, implement and complete its further clinical development and commercialization plans for fruquintinib and the timing of these events. In addition, as certain studies rely on the use of other drug products such as sintilimab as combination therapeutics with fruquintinib, such risks and uncertainties include assumptions regarding the safety, efficacy, supply and continued regulatory approval of these therapeutics. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the U.S. Securities and Exchange Commission, on The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
CONTACTS
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
HSBC |
Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
Cavendish |
Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
[1] The Global Cancer Observatory, kidney cancer fact sheet. https://gco.iarc.who.int/media/globocan/factsheets/cancers/29-kidney-fact-sheet.pdf. Accessed February 19, 2025.
[2] The Global Cancer Observatory, China fact sheet. https://gco.iarc.who.int/media/globocan/factsheets/populations/160-china-fact-sheet.pdf. Accessed February 19, 2025.
[3] Sun Q, et al. Discovery of fruquintinib, a potent and highly selective small molecule inhibitor of VEGFR 1, 2, 3 tyrosine kinases for cancer therapy. Cancer Biol Ther. 2014;15(12):1635-45. doi: 10.4161/15384047.2014.964087.
[4] Dasari NA, et al. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO‑2): an international, multicentre, randomised, double‑blind, Phase III study. Lancet. 2023;402(10395):41‑53. doi:10.1016/S0140‑6736(23)00772‑9.
[5] Li J, et al. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA. 2018;319(24):2486-2496. doi:10.1001/jama.2018.7855.
[6] Xu H, et al. Fruquintinib Plus Sintilimab in Patients with Treatment‑Naïve and Previously Treated Advanced Renal Cell Carcinoma: Results from a Phase Ib/II Clinical Trial. Targeted Oncology. 2025; 20:113–125. doi.org/10.1007/s11523-024-01120-6.
[7] Wang J, et al. Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. mAbs 2019;11(8): 1443-1451. doi: 10.1080/19420862.2019.1654303.
HUTCHMED (China) Limited (“HUTCHMED”) notes the below text, which is from a voluntary announcement released to the Stock Exchange of Hong Kong Limited on March 14, 2025. The text relates to the adoption of the 2025 Long Term Incentive Plan with effect from April 24, 2025.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
CONTACTS
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
HSBC |
Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
Cavendish |
Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
Hong Kong, Shanghai & Florham Park, NJ — Friday, March 14, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM: HCM; SEHK:13) announces that the non-performance based awards granted under the Long Term Incentive Plan (“LTIP”) on March 13, 2024 to the following person discharging managerial responsibilities were vested on March 13, 2025:-
| Award Holder | Number of ordinary shares | |
| Dr Weiguo Su (Executive Director, Chief Executive Officer and Chief Scientific Officer) | 19,913 |
The notification set out below is provided in accordance with the requirements of the UK Market Abuse Regulation.
Dr Weiguo Su
| 1 | Details of the person discharging managerial responsibilities/person closely associated | |||||
| a) | Name | Dr Weiguo Su | ||||
| 2 | Reason for the notification | |||||
| a) | Position/status | Executive Director, Chief Executive Officer and Chief Scientific Officer | ||||
| b) | Initial notification/Amendment | Initial notification | ||||
| 3 | Details of the issuer, emission allowance market participant, auction platform, auctioneer or auction monitor | |||||
| a) | Name | HUTCHMED (China) Limited | ||||
| b) | LEI | 2138006X34YDQ6OBYE79 | ||||
| 4 | Details of the transaction(s): section to be repeated for (i) each type of instrument; (ii) each type of transaction; (iii) each date; and (iv) each place where transactions have been conducted | |||||
| a) | Description of the financial instrument, type of instrument Identification code |
Ordinary Shares of US$0.10 Ordinary Share with DI ISIN: KYG4672N1016 |
||||
| b) | Nature of the transaction | Vesting of awards granted on March 13, 2024 under HUTCHMED’s LTIP | ||||
| c) | Price(s) and volume(s) |
|
||||
| d) | Aggregated information
|
N/A | ||||
| e) | Date of the transaction | 2025-03-13 | ||||
| f) | Place of the transaction | Outside a trading venue | ||||
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery, global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception, HUTCHMED has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
CONTACTS
| Investor Enquiries | +852 2121 8200 / ir@hutch-med.com |
| Media Enquiries | |
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
| Panmure Liberum | Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
| HSBC | Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
| Cavendish | Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
| Date: | Monday, March 31, 2025 |
| Time: | 3:00 pm Hong Kong Time (8:00 am London Time) |
| Location: | 47th Floor, Cheung Kong Center, 2 Queen’s Road Central, Hong Kong |
| Online: | https://meetings.computershare.com/Hutchmed2025EGM |
Hong Kong, Shanghai & Florham Park, NJ — Thursday, March 6, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that it has completed enrollment of its a Phase II trial of fanregratinib (HMPL-453) for intrahepatic cholangiocarcinoma (“IHCC”) patients with fibroblast growth factor receptor (“FGFR”)2 fusion/rearrangement.
The study is a single-arm, multi-center, open-label, Phase II registration study to evaluate the efficacy, safety and pharmacokinetic of fanregratinib in treating advanced IHCC patients with FGFR2 fusion/rearrangement. Primary endpoint is objective response rate (ORR). Secondary endpoints include progression-free survival (PFS), disease control rate (DCR), duration of response (DoR) and overall survival (OS). A total of 87 patients were enrolled into the registration phase of the study. Additional details may be found at clinicaltrials.gov using identifier NCT04353375.
The first patient received the first dose in March 2023 and HUTCHMED expects to announce topline results from the study around the end of 2025. If favorable, the results could enable a New Drug Application submission to China’s National Medical Products Administration (NMPA).
About Fanregratinib
Fanregratinib (HMPL‑453) is a novel, highly selective and potent inhibitor targeting FGFR 1, 2 and 3. Aberrant FGFR signaling has been found to be a driving force in tumor growth, promotion of angiogenesis and resistance to anti-tumor therapies. Abnormal FGFR gene alterations are believed to be the drivers of tumor cell proliferation in several solid tumor settings.
HUTCHMED currently retain all rights to fanregratinib worldwide.
About IHCC with FGFR2 Fusion/Rearrangement
IHCC is one of the subtypes of primary bile duct cancer. In China, an estimated 61,900 newly diagnosed IHCC occurred in 2015 and the overall IHCC incidence increased by 9.2% per year between 2006 and 2015.[1] FGFR2 fusion has been reported to have a prevalence of 10-15% in IHCC patients.[2],[3]
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the therapeutic potential of fanregratinib, the further clinical development for fanregratinib, its expectations as to whether any studies on fanregratinib would meet their primary or secondary endpoints, and its expectations as to the timing of the completion and the release of results from such studies. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding enrollment rates and the timing and availability of subjects meeting a study’s inclusion and exclusion criteria; changes to clinical protocols or regulatory requirements; unexpected adverse events or safety issues; the ability of fanregratinib, including as a combination therapy, to meet the primary or secondary endpoint of a study, to obtain regulatory approval in different jurisdictions and to gain commercial acceptance after obtaining regulatory approval; the potential market of fanregratinib for a targeted indication and the sufficiency of funding. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
CONTACTS
| Investor Enquiries | +852 2121 8200 / ir@hutch-med.com |
| Media Enquiries | |
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
| Panmure Liberum | Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
| HSBC | Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
| Cavendish | Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
[1] An L, Zheng R, Zhang S, et al. Hepatocellular carcinoma and intrahepatic cholangiocarcinoma incidence between 2006 and 2015 in China: estimates based on data from 188 population-based cancer registries. Hepatobiliary Surg Nutr. 2023 Feb 28;12(1):45-55.
[2] Arai Y, Totoki Y, Hosoda F, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology. 2014;59:1427–34.
[3] Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003–10.
Hong Kong, Shanghai & Florham Park, NJ — Wednesday, March 5, 2025: HUTCHMED (China) Limited (“HUTCHMED” or the “Company”) (Nasdaq/AIM:HCM, HKEX:13) today announces that Mr Wong Tak Wai (Mr Alvin Wong) is appointed as an Independent Non-executive Director and a member of the Audit Committee of the Company with effect from March 6, 2025.
Mr Wong has over 35 years of extensive experience in accounting, auditing and corporate finance. He has acted in a pivotal role in assisting companies with their stock exchange listings and has been instrumental in completing numerous mergers and acquisitions. After a distinguished career spanning more than three decades, Mr Wong retired as a partner of PricewaterhouseCoopers in 2017.
Dr Dan Eldar, Chairman of HUTCHMED, said “On behalf of the Board, I would like to extend a warm welcome to Mr Wong to the Company. We believe that his extensive experience in finance and accounting will greatly contribute to the strategic growth and financial stewardship of the Company.”
Mr Wong, aged 68, is currently a non-executive director of Melbourne Enterprises Limited (HKEX: 158). He was the president and a council member of the Hong Kong Institute of Certified Public Accountants (“HKICPA”), chairman of the HKICPA auditing standards committee, and a member of various committees of the International Federation of Accountants. He was also a member of the Sustainable Agricultural Development Fund Advisory Committee.
Mr Wong holds a Bachelor of Commerce degree from University of Otago, New Zealand and is a Fellow of the HKICPA and an Associate of the Institute of Chartered Accountants of Australia and New Zealand.
Mr Wong currently holds or has held in the past five years the following directorships / partnerships:
| Current Directorships / Partnerships: | Previous Directorships / Partnerships in the last five years: |
|
Chinachem Group Holdings Limited Melbourne Enterprises Limited Pondbury Investments Limited |
Estate Of Wang Teh Huei Ltd Estate Of Wang Teh Huei (No. 2) Ltd Vipin Limited Wyberton Limited |
Save for the appointments listed above, Mr Wong has held no other directorships or partnerships during the period of five years prior to his appointment as a director of HUTCHMED. He does not have any relationship with any Directors, senior management or substantial or controlling shareholders of HUTCHMED. Mr Wong has confirmed (a) his independence as regards each of the factors for independence referred to in Rule 3.13(1) to (8) of the Rules Governing the Listing of Securities on The Stock Exchange of Hong Kong Limited (“HK Listing Rules”); (b) that he had no past or present financial or other interest in the business of the Company or its subsidiaries or any connection with any core connected person (as defined in the HK Listing Rules) of the Company; and (c) that there are no other factors that may affect his independence at the time of his appointment.
The initial term of the appointment of Mr Wong as an Independent Non-executive Director of the Company shall end at the next following annual general meeting of the Company, subject to retirement in accordance with the Articles of Association of the Company and applicable legal and regulatory requirements, and thereafter for successive periods of 12 months, unless he is not re-elected at the next following annual general meeting or his appointment is otherwise terminated earlier by either party in writing. The director’s fees of Mr Wong as an Independent Non-executive Director and member of the Audit Committee of the Company under his appointment letter are US$76,000 and US$13,500 per annum respectively, which were determined by the Board with reference to the director’s duties and responsibilities and prevailing market conditions. Such fees are subject to review from time to time and proration for any incomplete year of service.
Mr Wong does not have any interest in any shares of the Company within the meaning of Part XV of the Securities and Futures Ordinance (Cap. 571 of the Laws of Hong Kong).
Save for the information disclosed above, there is no other information in relation to Mr Wong that is required to be disclosed pursuant to Rule 17 and Schedule 2(g) of the AІM Rules for Companies or Rule 13.51(2) of the HK Listing Rules, and there are no other matters concerning the appointment of Mr Wong that are required to be brought to the attention of the shareholders of HUTCHMED.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery, global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception, HUTCHMED has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This announcement contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, the risk that current or future appointees to HUTCHMED’s board of directors are not effective in their respective positions, the difficulty in locating and recruiting suitable candidates for its board of directors and the management difficulties which may arise from changes in HUTCHMED’s board of directors. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, on AІM and with The Stock Exchange of Hong Kong Limited. HUTCHMED undertakes no obligation to update or revise the information contained in this announcement, whether as a result of new information, future events or circumstances or otherwise.
CONTACTS
| Investor Enquiries | +852 2121 8200 / ir@hutch-med.com |
| Media Enquiries | |
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
| Panmure Liberum | Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
| HSBC | Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
| Cavendish | Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
HUTCHMED (China) Limited (“HUTCHMED”) notes the below text, which is from an announcement released to the Stock Exchange of Hong Kong Limited on March 2, 2025 pursuant to Chapter 14 of the Rules Governing the Listing of Securities on The Stock Exchange of Hong Kong Limited.
The announcement provides an update on the proposed divestment of HUTCHMED’s 45% equity interest in Shanghai Hutchison Pharmaceuticals Limited (“SHPL”) as described in the RNS announcements dated January 2, 2025. As previously announced, the transaction includes the proposed disposal of shares representing a 35% equity interest in SHPL to GP Health Service Capital Co., Ltd (“GP Health Service Capital”), which in turn has the right to designate (i) a party to purchase no more than 10% equity interest in SHPL, and (ii) a fund established by GP Health Service Capital as manager and one of the general partners to purchase the remaining shares.
The below text describes the investment funds that have been designated by GP Health Service Capital, and provides an update on the date when the Extraordinary General Meeting Circular is expected to be dispatched to shareholders.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
CONTACTS
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
HSBC |
Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
Cavendish |
Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
HUTCHMED (China) Limited (“HUTCHMED”) notes the below text, which is from an announcement released to the Stock Exchange of Hong Kong Limited on February 28, 2025 pursuant to Chapter 14 of the Rules Governing the Listing of Securities on The Stock Exchange of Hong Kong Limited. The text relates to the further update on dispatch timing of the extraordinary general meeting circular that relates to the transaction described in the announcement dated January 2, 2025, entitled “HUTCHMED Announces US$608 million Divestment of Non-Core Joint Venture”.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
CONTACTS
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
HSBC |
Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
Cavendish |
Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
Hong Kong, Shanghai & Florham Park, NJ — Wednesday, February 19, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM: HCM; SEHK:13) will be announcing its final results for the year ended December 31, 2024 on Wednesday, March 19, 2025 at 7:00 am Eastern Daylight Time (EDT) / 11:00 am Greenwich Mean Time (GMT) / 7:00 pm Hong Kong Time (HKT).
Analysts and investors are invited to join a conference call and audio webcast presentation with Q&A, conducted by HUTCHMED management.
The English conference call and audio webcast will take place at 8:00 am EDT / 12:00 pm GMT / 8:00 pm HKT on Wednesday, March 19, 2025. In addition to the usual English webcast, there will also be a Chinese (Putonghua) webcast at 12:30 am GMT / 8:30 am HKT on Thursday, March 20, 2025 (8:30 pm EDT on Wednesday, March 19, 2025). Both webcasts will be available live via the company website at www.hutch-med.com/event/. The presentation will be available for downloading before the conference call begins. Details of the conference call dial-in will be provided in the financial results announcement and on the company website. A replay will also be available on the website shortly after the event.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception, HUTCHMED has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
CONTACTS
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
| HSBC | Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
| Cavendish | Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
| Job Title: | Senior Scientist I for Oncology Research |
| Location: | Hong Kong |
Key Responsibilities
- Design and perform molecular and cell biology experiments, analyze data and draft study report
- Summary and presentation of study findings at internal or external meetings
- Research of tumor biomarkers, mechanism of action of new anti-cancer products and exploration of new indications or drug combinations
- Literature review and raise the proposal of potential new targets for cancer therapy
- Coordinate internal resource or outsourcing services to ensure timely delivery of study results
Qualifications
- PhD in biology, biochemistry or related field, or a master’s degree for at least 3 years of industry working experience
- A track record experience in cancer research
- Expertise of molecular biology and cell biology experiments, including cell culture, western blot, RT-PCR and flow cytometry
- Knowledge of NGS, bioinformatics, experience with in vivo animal studies or pathology experiment is a plus
- A self-motivated scientist, attention to detail, and ability to think independently
- Ability to work under pressure and deliver on time
- Good interpersonal skill, good oral and written communication skills, fluent in English and Mandarin Chinese
If interested, please forward your resume to recruit@hutch-med.com.
| Job Title: | Senior Technician for Oncology Research |
| Location: | Hong Kong |
Key Responsibilities
- Daily operation and maintenance of laboratory instruments and equipment
- Order and storage of experiment expendable, reagents and materials
- Cell bank maintenance, including cell thawing, culturing, freezing procedures and documents
- Perform or support a variety of biology experiments
- Ensure strict adherence to relevant safety procedures
Qualifications
- Bachelor’s degree in Biochemistry, Biotechnology, Life Sciences or related field, with working history in industry preferred
- Technical expertise in the following: cell culture, cell viability assay, kinase assay, etc.
- Data recording, analysis and troubleshooting capabilities
- Strong sense of responsibility and teamwork, well communication with the team
- Fluent in spoken Mandarin is a plus
If interested, please forward your resume to recruit@hutch-med.com.