- Hutchmed
- | Announcements & Press Releases
Hong Kong, Shanghai, & Florham Park, NJ: Monday, March 31, 2025: HUTCHMED (China) Limited (“HUTCHMED” or the “Company”) (Nasdaq/AIM: HCM; HKEX:13) today announces that the ordinary resolution put to its Extraordinary General Meeting (“EGM”) held on March 31, 2025 was duly passed.
Reference is made to the notice of EGM dated March 14, 2025 and the circular to shareholders dated March 14, 2025 (the “Circular”) issued by the Company. Unless otherwise defined herein, capitalized terms used in this announcement shall have the same meanings as those defined in the Circular.
The poll results of the ordinary resolution were as follows:
| Ordinary Resolution | Number of Votes (%)* | Passed by Shareholders | ||
| For | Against | Withheld# | ||
| To approve the transactions related to the sale and purchase of a total of 45% equity interest in Shanghai Hutchison Pharmaceuticals Limited under various agreements, and all actions by the Company and/or its subsidiaries pursuant or incidental to such transactions.^ |
475,229,253 (99.9745%) |
121,270 (0.0255%) |
1,670 | Yes |
* Percentages rounded to 4 decimal places.
# A vote withheld is not a vote in law and is not counted in the calculation of the proportion of the votes for or against a resolution.
^ The full text of the resolution is set out in the notice of EGM dated March 14, 2025.
Notes:
- Except for Ms Ling YANG who had prior overseas commitments and was unable to attend the EGM, all directors of the Company, namely Dr Dan ELDAR, Dr Weiguo SU, Mr CHENG Chig Fung, Johnny, Ms Edith SHIH, Mr Paul Rutherford CARTER, Dr Renu BHATIA, Dr Chaohong HU, Mr Graeme Allan JACK, Professor MOK Shu Kam, Tony and Mr WONG Tak Wai, attended the EGM, either in person or by means of electronic facilities.
- Number of shares entitling the holders to attend and vote on the resolution at the EGM: 871,601,095 shares.
- Number of shares entitling the holders to attend and abstain from voting in favor as set out in Rule 13.40 of the Rules Governing the Listing of Securities on The Stock Exchange of Hong Kong Limited (the “Listing Rules”) at the EGM: Nil.
- Number of shares for holders required under the Listing Rules to abstain from voting at the EGM: Nil.
- The scrutineer for the poll at the EGM was Computershare Investor Services (Jersey) Limited, the Principal Share Registrar of the Company.
About HUTCHMED
HUTCHMED (Nasdaq/AIM: HCM; HKEX: 13) is an innovative, commercial-stage, biopharmaceutical company. Іt is committed to the discovery, global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, and the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
CONTACTS
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
| HSBC | Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
| Cavendish | Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
— First and only EZH2 inhibitor approved by the NMPA —
—HUTCHMED’s fourth product, and its first approval in hematological malignancies —
Hong Kong, Shanghai & Florham Park, NJ — Friday, March 21, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that the New Drug Application (“NDA”) for TAZVERIK® (tazemetostat) has been granted conditional approval in China for the treatment of adult patients with relapsed or refractory (“R/R”) follicular lymphoma (“FL”) with EZH2 mutation who have received at least two prior systemic therapies. This approval follows the priority review status by the National Medical Products Administration (“NMPA”) and marks the first nationwide regulatory approval for TAZVERIK® in China.
The conditional approval by the NMPA was supported by results from a multicenter, open-label, Phase II bridging study in China, and clinical studies conducted by Epizyme, Inc. (“Epizyme”), an Ipsen company, outside China. The primary objective of the bridging study is to evaluate the objective response rate (“ORR”) of TAZVERIK® for the treatment of patients with R/R FL whose disease harbor EZH2 mutations. The secondary objectives included duration of response (“DoR”), progression-free survival (PFS), and overall survival (OS) of TAZVERIK® for the treatment of R/R FL patients, as well as to evaluate the safety and pharmacokinetics. Additional details can be found at clinicaltrials.gov, using identifier NCT05467943.
“This approval represents a significant advancement in the management of this challenging disease. The majority of FL patients experience multiple relapses over their lifetime, posing substantial treatment difficulties and often leading to poor outcomes,” said Dr Junning Cao of Fudan University Cancer Center and the lead principal investigator of the bridging study. “TAZVERIK® has demonstrated promising efficacy in patients harboring EZH2 mutation in clinical trials. We are eager to provide this transformational epigenetic therapy to patients in China who have long sought new effective treatment options.”
“We are thrilled to be able to bring this innovative EZH2 inhibitor to patients in China. This approval highlights our dedication to addressing unmet medical needs not only through our internal pipeline, but also through partnering,” said Dr Michael Shi, Head of R&D and Chief Medical Officer of HUTCHMED. “It also marks our first approval in hematological malignancies, unveiling a new chapter for HUTCHMED as we extend our footprint into this disease area. As we move forward, we are dedicated to making this product available to R/R FL patients as soon as possible and will continue striving to make a meaningful impact on the lives of more patients suffering from devastating diseases.”
TAZVERIK® is a first-in-class methyltransferase inhibitor of EZH2 developed by Epizyme. It is approved by the US Food and Drug Administration (“FDA”) for the treatment of certain patients with R/R FL and certain patients with advanced epithelioid sarcoma (“ES”) under the FDA accelerated approval program. It is also approved by the Japan Ministry of Health, Labour and Welfare (MHLW) for certain patients with R/R FL. In 2021, HUTCHMED and Epizyme entered a strategic partnership. HUTCHMED is responsible for the research, development, manufacturing and commercialization of TAZVERIK® in China Mainland, Hong Kong, Macau and Taiwan. Epizyme will be the Marketing Authorization Holder of TAZVERIK® in China.
TAZVERIK® was approved for use in the Hainan Boao Lecheng International Medical Tourism Pilot Zone (Hainan Pilot Zone) in May 2022, under the Clinically Urgently Needed Imported Drugs scheme, for the treatment of certain patients with ES and FL consistent with the label as approved by the FDA. TAZVERIK® was approved in Macau in March 2023 and in Hong Kong in May 2024.
The ongoing SYMPHONY-1 study will serve as the confirmatory trial to validate the clinical benefits of TAZVERIK®. SYMPHONY-1 is an international, multicenter, randomized, double-blind, active-controlled, 3-stage, biomarker-enriched, confirmatory Phase Ib/III study, which is designed to evaluate the safety and efficacy of TAZVERIK® in combination with rituximab and lenalidomide (R²) in patients with R/R FL after at least one prior line of therapy (NCT04224493). Epizyme is the sponsor of SYMPHONY-1 and HUTCHMED is leading the study in China.
About Follicular Lymphoma
FL is the second most common subtype of non-Hodgkin’s lymphoma (“NHL”), making up 20-30% of all NHL. In 2022, there were an estimated 81,000 and 78,000 new cases of NHL in China and the US, respectively.[1],[2],[3]
About Tazemetostat approval in the United States and Japan
Tazemetostat is a methyltransferase inhibitor indicated in the United States for the treatment of:
- Adults and pediatric patients aged 16 years and older with metastatic or locally advanced ES not eligible for complete resection.
- Adult patients with R/R FL whose tumors are positive for an EZH2 mutation as detected by an FDA-approved test and who have received at least two prior systemic therapies.
- Adult patients with R/R FL who have no satisfactory alternative treatment options.
These indications are approved under accelerated approval by the US FDA based on ORR and DoR. Continued approval for these indications may be contingent upon verification and description of clinical benefit in confirmatory trials.
The most common (≥20%) adverse reactions in patients with ES are pain, fatigue, nausea, decreased appetite, vomiting and constipation. The most common (≥20%) adverse reactions in patients with FL are fatigue, upper respiratory tract infection, musculoskeletal pain, nausea and abdominal pain.
Please see the US Full Prescribing Information for TAZVERIK® (tazemetostat).
TAZVERIK® is approved in Japan with the indication of relapsed or refractory EZH2 gene mutation-positive FL (only when standard treatment is not applicable).
TAZVERIK® is commercialized by Epizyme in the US and by Eisai in Japan.
TAZVERIK® is a registered trademark of Epizyme Inc., an Ipsen company.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the therapeutic potential of tazemetostat for the treatment of patients with relapsed or refractory follicular lymphoma and the further clinical development of tazemetostat in this and other indications. Forward‑looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding the sufficiency of clinical data to support NDA approval of tazemetostat for the treatment of patients with follicular lymphoma in China and other jurisdictions, the safety profile of tazemetostat, HUTCHMED’s ability to fund, implement and complete its further clinical development and commercialization plans for tazemetostat, and the timing of these events. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
CONTACTS
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
HSBC |
Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
Cavendish |
Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
[1] NCCN Guidelines for Patients: Follicular Lymphoma. https://www.nccn.org/patients/guidelines/content/PDF/nhl-follicular-patient.pdf. Accessed February 17, 2025.
[2] SEER Cancer Stat Facts: Follicular Lymphoma. National Cancer Institute. https://seer.cancer.gov/statfacts/html/follicular.html. Accessed February 17, 2025
[3] The Global Cancer Observatory (GLOBOCAN), Cancer Today | IARC. https://gco.iarc.who.int/today/en/dataviz/bars?cancers=34. Accessed February 17, 2025.
Hong Kong, Shanghai & Florham Park, NJ — Thursday, March 20, 2025: HUTCHMED (China) Limited (“HUTCHMED” or the “Company”) (Nasdaq/AIM:HCM, HKEX:13) today announces that Mr Paul Rutherford Carter and Mr Graeme Allan Jack, who have both served as Independent Non-executive Directors of the Company for more than eight years, have informed the Company that they would not seek re-election after retiring from the Board at the forthcoming annual general meeting of the Company to be held on May 13, 2025 (“AGM”). Consequently, both will cease to be Independent Non-executive Directors of the Company at the conclusion of the AGM. Upon their retirement, they will also step down from their roles as chairmen and members of the board committees of the Company.
In connection with the intended retirement of the above Directors, the Board has approved the following changes to the composition of the board committees and Senior and Lead Independent Non-executive Director of the Company, effective from the conclusion of the AGM, subject to the respective Directors being re-elected as Directors by the shareholders at the AGM:-
- Professor Mok Shu Kam, Tony will be appointed as Senior and Lead Independent Non-executive Director;
- Mr Wong Tak Wai will be appointed as the chairman of the Audit Committee and a member of the Remuneration Committee;
- Dr Chaohong Hu will be appointed as a member of the Audit Committee; and
- Dr Renu Bhatia will be appointed as the chairman of the Remuneration Committee.
Each of Professor Mok, Mr Wong, Dr Hu and Dr Bhatia is currently an Independent Non-executive Director of the Company.
Dr Dan Eldar, the Chairman of HUTCHMED, said “Mr Carter, who has served as the Senior Independent Non-executive Director and the chairman of the Remuneration Committee, has played a pivotal role in shaping the remuneration policies and practices of the Company. His leadership and guidance have been crucial in retaining and motivating a broader and more diverse pool of employees of the highest caliber and experience needed to shape and execute the strategy of the Company. The Board extends its appreciation to Mr Carter for his outstanding service and contributions to the success of the Company.”
Dr Eldar continued, “Mr Jack, who has served as the chairman of the Audit Committee, has been instrumental in overseeing the financial reporting and audit processes of the Company, ensuring the highest standards of integrity and transparency. The Board expresses its deepest gratitude to Mr Jack for his invaluable contributions and dedication to the Company. We wish them both all the best in their future endeavors.”
Pursuant to the requirements of Rule 13.51(2) of the HK Listing Rules, each of Mr Carter and Mr Jack have confirmed that he has no disagreement with the Board and that there are no other matters that need to be brought to the attention of the shareholders of the Company in connection with his retirement from the Board.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery, global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, and the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This announcement contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, the risk that current or future appointees to HUTCHMED’s board of directors are not effective in their respective positions, the difficulty in locating and recruiting suitable candidates for its board of directors and the management difficulties which may arise from changes in HUTCHMED’s board of directors. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, on AІM and with The Stock Exchange of Hong Kong Limited. HUTCHMED undertakes no obligation to update or revise the information contained in this announcement, whether as a result of new information, future events or circumstances or otherwise.
CONTACTS
| Investor Enquiries | +852 2121 8200 / ir@hutch-med.com |
| Media Enquiries | |
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
| Panmure Liberum | Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
| HSBC | Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
| Cavendish | Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
— SAVANNAH Phase II trial demonstrated high and durable response rates with savolitinib plus TAGRISSO® in MET-high lung cancer, representing a promising chemo-free oral treatment strategy to address mechanisms of resistance in the advanced setting —
— Long-term survival benefit and safety observed in savolitinib Phase IIIb study in METex14 NSCLC —
Hong Kong, Shanghai & Florham Park, NJ — Thursday, March 20, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that new and updated data from several studies of compounds discovered by HUTCHMED, savolitinib and surufatinib, will be presented at the European Lung Cancer Congress (ELCC) 2025, taking place on March 26-29, 2025 in Paris, France.
| Title: | SAVANNAH: Savolitinib (savo) + osimertinib (osi) in patients (pts) with EGFRm advanced NSCLC and MET overexpression (OverExp) and/or amplification (Amp) following progressive disease (PD) on osi |
| Lead Author: | Myung-Ju Ahn, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea |
| Session: | Proffered Paper session 1 |
| Abstract Number: | #2O |
| Date & Time: | Wednesday, March 26, 2025,16:45 Central European Time |
| Location: | South Paris Room |
Results from the SAVANNAH Phase II trial (NCT03778229) showed savolitinib (300mg BID[1]) plus TAGRISSO® demonstrated a clinically meaningful and durable objective response rate (“ORR”) in patients with epidermal growth factor receptor mutated (“EGFRm”) non-small cell lung cancer (“NSCLC”) with high levels of MET overexpression and/or amplification whose disease progressed on treatment with first line TAGRISSO®.
Savolitinib plus TAGRISSO® demonstrated confirmed ORR of 56% (95% CI[2]: 45%–67%) and 55% (95% CI: 43%–66%), median duration of response (“DoR”) of 7.1 (95% CI: 5.6–9.6) and 9.9 (95% CI: 6.0–13.7) months, and median progression-free survival (“PFS”) of 7.4 (95% CI: 5.5–7.6) and 7.5 (95% CI: 6.4–11.3) months by investigator and blinded independent central review (BICR) assessment, respectively.
Safety results and discontinuation rates due to adverse events were consistent with the established profiles of each medicine and no new safety concerns were reported. In all patients treated with savolitinib (300mg BID) plus TAGRISSO®, Grade 3 or higher adverse events (AEs) occurred in 57% and Grade 3 or higher treatment related adverse events (TRAEs) occurred in 32% of the patients.
Savolitinib is an oral, potent and highly selective MET tyrosine kinase inhibitor (“TKI”) being jointly developed and commercialized by AstraZeneca and HUTCHMED. In 2023, savolitinib and TAGRISSO® received Fast Track Designation from the US Food and Drug Administration (FDA) in this setting.
| Title: | Final Overall Survival and Long-term Safety Outcomes of Savolitinib in Patients with Locally Advanced or Metastatic NSCLC Harboring MET Exon 14 (METex14) Mutation: An Update from a Phase 3b Study |
| Lead Author: | Yongfeng Yu, Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China |
| Session: | Poster Display session |
| Abstract Number: | #80P |
| Date & Time: | Friday March 28, 2025,13:00 Central European Time |
| Location: | Poster area |
Updated results from the savolitinib Phase IIIb study in China demonstrated survival benefits and long-term safety in MET exon 14 skipping alteration NSCLC, particularly in treatment-naïve patients (NCT04923945). Among the 166 patients who received savolitinib treatment, the median follow-ups for 87 treatment-naïve patients and 79 previously treated patients were 34.5 and 25.1 months, respectively. In 87 treatment-naïve patients, median overall survival (“OS”) was 28.3 months (95% CI: 17.5–not evaluable), and the 36-month OS rate was 44.7%. In 79 previously treated patients, median OS was 25.3 months (95% CI: 20.5–30.5), and the 24-month OS rate was 51.7%. Post-hoc OS subgroup analysis suggested that patients with baseline brain metastasis also gain survival benefit with median OS of 15.3 months and 25.3 months in treatment-naïve patients (10/87 patients) and previously treated (21/79 patients) with brain metastasis, respectively. No new safety signal was observed.
Savolitinib is approved in China under the brand name ORPATHYS® for this patient population.
| Title: | Final overall survival (OS) of surufatinib plus PD-1/PD-L1 antibodies as maintenance therapy following first line (1L) platinum-based chemotherapy (Chemo) plus PD-1/PD-L1 antibodies in patients (pts) with extensive-stage small cell lung cancer (ES-SCLC) |
| Lead Author: | Yi Hu, Chinese PLA General Hospital, Beijing, China |
| Session: | Poster Display session |
| Abstract Number: | #310P |
| Date & Time: | Friday March 28, 2025,13:00 Central European Time |
| Location: | Poster area |
Results from the exploratory study suggests surufatinib plus immunotherapy as maintenance therapy following first line chemo‑immunotherapy demonstrated durable survival benefit for patients with extensive-stage small cell lung cancer (“SCLC”) patients (NCT05509699). At data cut-off on July 31, 2024, a total of 21 patients were enrolled in this single arm Phase IIa part of the study and received at least one dose of surufatinib plus PD‑1/PD‑L1 antibodies treatment. The median follow-up duration was 17.1 months for maintenance and 22.5 months for first line (induction + maintenance) therapy. The 12-month and 18-month OS rates were both 57.1% for maintenance therapy; and 85.7% and 57.1% for first line therapy.
About NSCLC and SAVANNAH
Lung cancer is the leading cause of cancer death among men and women, accounting for about one-fifth of all cancer deaths. Lung cancer is broadly split into SCLC or NSCLC, the latter accounting for about 80% of cases. Approximately 10 to 15% of patients with NSCLC in the US and Europe and 30 to 40% of patients in Asia have an epidermal growth factor receptor (“EGFR”) mutation. While EGFR TKIs have significantly improved outcomes in the first line setting, treatment resistance and disease progression are extremely common, and a significant unmet need exists in later-line settings for effective and well-tolerated treatment options.
SAVANNAH is an ongoing randomized, global Phase II trial studying the efficacy of savolitinib added to TAGRISSO® in patients with EGFRm, locally advanced or metastatic NSCLC with MET overexpression and/or amplification who progressed following treatment with TAGRISSO®. Based on the original single-arm trial design, patients were treated with savolitinib 300 or 600mg QD[3] or 300mg BID, in combination with oral TAGRISSO® 80mg QD. In 2022, a comparison of savolitinib 300 mg BID and TAGRISSO® 80 mg QD to savolitinib 300mg BID and placebo was added to the trial to evaluate contribution of components.
The trial enrolled over 360 patients in more than 80 centers globally, including in North America, Europe, South America and Asia. The primary endpoint is ORR and key secondary endpoints include PFS and DoR.
In August 2022, positive interim results from the SAVANNAH trial were presented at the International Association for the Study of Lung Cancer 2022 World Conference on Lung Cancer (WCLC).
The global SAFFRON Phase III trial is currently ongoing to further assess the savolitinib plus TAGRISSO® combination versus platinum-based doublet chemotherapy in patients with EGFRm, MET-overexpressed and/or amplified, locally advanced or metastatic NSCLC following TAGRISSO®. Patients are being prospectively selected using the high MET level cut-off identified in SAVANNAH.
About Savolitinib
Savolitinib is an oral, potent, and highly selective MET TKI that has demonstrated clinical activity in advanced solid tumors. MET is a tyrosine kinase receptor that has an essential role in normal cell development. Savolitinib blocks atypical activation of the MET receptor tyrosine kinase pathway that occurs because of mutations (such as exon 14 skipping alterations or other point mutations), gene amplification or protein overexpression. MET overexpression and/or amplification can lead to tumor growth and the metastatic progression of cancer cells, and is a known mechanism of acquired resistance to EGFR TKIs. The prevalence of MET depends on the sample type, detection method and assay cut-off used.
Savolitinib is approved in China and is marketed under the brand name ORPATHYS® by our partner, AstraZeneca, for the treatment of adult patients with locally advanced or metastatic NSCLC with MET exon 14 skipping alteration, representing the first selective MET inhibitor approved in China. It has been included in the National Reimbursement Drug List of China (“NRDL”) since March 2023.
It is currently under clinical development for multiple tumor types, including lung, kidney, and gastric cancers as a single treatment and in combination with other medicines.
About Surufatinib
Surufatinib is a novel, oral angio-immuno kinase inhibitor that selectively inhibits the tyrosine kinase activity associated with vascular endothelial growth factor receptors (VEGFRs) and fibroblast growth factor receptor (FGFR), which both inhibit angiogenesis, and colony stimulating factor-1 receptor (CSF-1R), which regulates tumor-associated macrophages, promoting the body’s immune response against tumor cells. Its unique dual mechanism of action may be very suitable for possible combinations with other immunotherapies, where there may be synergistic anti-tumor effects.
Surufatinib is marketed in China by HUTCHMED under the brand name SULANDA®, and was first included in the NRDL in January 2022 for the treatment of non-pancreatic and pancreatic neuroendocrine tumors (NETs).
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery, global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception, it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, and the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit www.hutch‑med.com or follow us on LinkedIn.
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the therapeutic potential of the savolitinib or surufatinib for the treatment of patients with lung cancer and the further clinical development of the savolitinib or surufatinib in this and other indications. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding the timing and outcome of clinical studies and the sufficiency of clinical data to support New Drug Application approval of the savolitinib or surufatinib in lung cancer or other indications in the US, China or other jurisdictions, its potential to gain approvals from regulatory authorities on an expedited basis or at all, the safety profile of savolitinib or surufatinib, HUTCHMED’s ability to fund, implement and complete its further clinical development and commercialization plans for savolitinib or surufatinib and the timing of these events. In addition, as certain studies rely on the use of other drug products such as osimertinib as combination therapeutics with savolitinib and PD-1/PD-L1 antibodies as combination therapeutics with surufatinib, such risks and uncertainties include assumptions regarding the safety, efficacy, supply and continued regulatory approval of these therapeutics. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the U.S. Securities and Exchange Commission, on AIM and on The Stock Exchange of Hong Kong Limited. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
CONTACTS
| Investor Enquiries | +852 2121 8200 / ir@hutch-med.com |
| Media Enquiries | |
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
| Panmure Liberum | Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
| HSBC | Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
| Cavendish | Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
REFERENENCES
[1] BID = Twice per day.
[2] CI = Confidence interval.
[3] QD = Once per day.
65% oncology products revenue growth drove profitable operation and supported new ATTC platform
Hong Kong, Shanghai & Florham Park, NJ — Wednesday, March 19, 2025: HUTCHMED (China) Limited (“HUTCHMED”, the “Company” or “we”) (HKEX:13; Nasdaq/AIM:HCM) today reports its financial results for the year ended December 31, 2024 and provides updates on key clinical and commercial developments.
HUTCHMED to host results webcasts today at 8:00 a.m. EDT / 12:00 noon GMT / 8:00 p.m. HKT in English on Wednesday, March 19, 2025, and tomorrow at 8:30 a.m. HKT in Chinese (Putonghua) on Thursday, March 20, 2025. After registration, investors may access the live webcast via HUTCHMED’s website at www.hutch-med.com/event.
All amounts are expressed in US dollars unless otherwise stated.
Global commercial progress and delivery of sustainable growth
- FRUZAQLA® (fruquintinib) ex-China in-market sales1 of $290.6 million in 2024 by Takeda, sustaining momentum in its first full year driven by rapid US patient uptake, and EU and Japan launches, triggering a sales milestone from Takeda2. Total oncology products in-market sales up 134% to $501.0 million.
- Consolidated revenue from oncology products of $271.5 million, up 65%.
- Net income of $37.7 million was achieved in 2024, with a cash balance of $836.1 million as of December 31, 2024, achieving financial self-reliance ahead of schedule.
- Agreed partial disposal of equity in SHPL3 joint venture for $608 million.
Pipeline progress and new technology platform
- Primary endpoint met in SACHI China Phase III interim analysis for savolitinib for EGFRm4 NSCLC5 with MET amplification, followed by swift NDA6 filing, acceptance and priority review granted by the NMPA7.
- Positive SAVANNAH global pivotal Phase II results for savolitinib in combination with TAGRISSO® for EGFRm NSCLC patients that progressed on TAGRISSO® treatment with MET overexpression or amplification, achieving high, clinically meaningful and durable response rate and shared with global regulatory authorities by AstraZeneca8.
- Positive FRUSICA-2 China Phase III results for fruquintinib with sintilimab in 2L9 RCC10.
- Presented ESLIM-01 China Phase III data at ASH11 and EHA12, highlighting strong, sustained, and long-term durable response rates of sovleplenib for ITP13 patients, with the NDA under review by the NMPA. Additional data were requested by CDE14 and subsequently submitted by HUTCHMED. Review of the supplementary data is currently under review by CDE.
- FRUSICA-1 Phase II results presented at ASCO15, leading to NMPA approval of a second indication of ELUNATE® (fruquintinib) for EMC16 with pMMR17
- First candidates from new ATTC18 platform, starting development of a new wave of drug candidates potentially more selective and tolerable than previous generations of antibody drug conjugates.
Dr Dan Eldar, Non-executive Chairman of HUTCHMED, said, “The successful commercialization of FRUZAQLA® outside of China by our partner Takeda and the resulting milestones achieved during the year were pivotal in helping HUTCHMED reach its profitability goals. I am proud that, at times of uncertainty in the global environment and in the capital markets, we have successfully established an independent ability to support our valuable discovery engine and development pipeline while mitigating operational risks. We expect to continue our global growth with further sales in the US and in other regions of the world, while continuing to develop our pipeline in new and promising directions. The long-term interests of our shareholders and benefits to patients around the world will always remain our top priorities.”
“At the end of 2024, we decided to dispose of our 45% equity interest in SHPL for $608 million, subject to closing conditions. I would like to take this opportunity to express my appreciation to the management team at SHPL for their contribution to its impressive growth over the last 20 years, which has delivered consistent benefits to consumers and shareholders alike. The commercial success and monetary contribution were important in supporting HUTCHMED’s novel drug R&D19, helping us to weather challenges in our industry as we developed innovative medicines for patients in need. As our innovative drugs business has become more self-reliant, we believe it is time for HUTCHMED to move on to our next phase of evolution, particularly as we focus on global clinical development of our ATTCs. The proceeds from the SHPL disposal, on top of the ongoing profits of our globally commercialized portfolio, enables us to expedite the roll-out of this differentiated platform, which will be key to our long-term value creation.”
Dr Weiguo Su, Chief Executive Officer and Chief Scientific Officer of HUTCHMED, said, “We’ve had a highly successful year, delivering against our strategy, in the clinic and commercially with our transformational medicines. This has culminated in HUTCHMED reaching profitability, which has been a key focus of ours. I’d like to thank and congratulate the team for this milestone, as we turn our attention to further growth and cultivating HUTCHMED’s next wave of medicines through our ATTC platform.”
“Our pioneering ATTC platform turns a new page in HUTCHMED’s innovative drug development story, establishing a new frontier in antibody-drug conjugates. This new portfolio of molecules is well placed to target a wide range of oncology indications with sizable market potential, including in first-line combinations. With the expertise and the financial strength to execute global clinical trials, we plan to move expeditiously into clinical development this year.”
“Our commercial medicines hit new milestones and expanded clinical development, reaching more patients in need around the world. Fruquintinib is now treating colorectal cancer patients in over a dozen countries, with more to come. FRUZAQLA® in-market sales exceeded $200 million within a year of launch, triggering the first sales milestone. In China, it was approved in second-line endometrial cancer, with average duration of treatment almost double that of fruquintinib’s first indication, and a third registrational study FRUSICA-2 has read out positively in kidney cancer.”
“For savolitinib, positive data from SACHI interim analysis in patients progressed on first line EGFR20 TKI21 treatment with MET amplification led us to file a NDA in China, which was accepted and granted priority review. We are hopeful that SAVANNAH/SAFFRON trials will support bringing this innovative medicine to patients globally. With recent full approval in both first-line and second-line MET exon 14 skipping alteration lung cancer, savolitinib remains one of the best-in-class medicines. A registration-intent study in MET-amplified gastric cancer is currently enrolling in China. We look forward to potentially expanding its indication as the first medicine for MET amplified EGFRm NSCLC and gastric cancer. Our marketed medicines will continue to support the revenue and earnings growth of HUTCHMED.”
“ESLIM-01 data for sovleplenib was presented at EHA and ASH, with durable response rate of 51.4% and overall response rate of 81.0%, significantly better than many different modalities of ITP medicines under development. These clinical results of sovleplenib again illustrate HUTCHMED’s R&D competency in selectivity, resulting in desirable efficacy and safety. We are working closely with the NMPA and look forward to bringing this innovative medicine to patients in need. ESLIM-02 registration Phase III in warm AIHA22 patients is enrolling and on-track to read out next year. A NDA is under review in China for tazemetostat for recurrent/refractory follicular lymphoma and approval is expected by mid-2025. We look forward to being able to add sovleplenib and tazemetostat to our commercial portfolio and their contributions to HUTCHMED’s continued growth.”
2024 FULL YEAR RESULTS & BUSINESS UPDATES
I. COMMERCIAL OPERATIONS
Oncology product in-market sales were up 134% (136% at CER23) to $501.0 million in 2024 (2023: $213.6m), leading to strong growth in oncology product consolidated revenue of 65% (67% at CER) to $271.5 million (2023: $164.2m).
- FRUZAQLA® (fruquintinib ex-China) in-market sales were $290.6 million in 2024 (2023: $15.1m) by Takeda, with strong performance reflecting rapid US patient uptake, as well as launches in over a dozen countries. Reaching $200.0 million sales triggered a $20 million milestone payment from Takeda.
- ELUNATE® (fruquintinib China) in-market sales increased 7% (9% at CER) to $115.0 million in 2024 (2023: $107.5m), maintaining its leading market share position in metastatic CRC24 and demonstrating resilience against rising pressure from competing products and their generics. New indication for EMC was approved in December 2024.
- SULANDA® (surufatinib) in-market sales increased 12% (14% at CER) to $49.0 million in 2024 (2023: $43.9m), as increasing brand awareness amongst doctors and improving NET25 diagnosis drives prescription growth and market share to 27% in 2024 (2023: 21%).
- ORPATHYS® (savolitinib) in-market sales approximated prior year (-2%, flat at CER) to $45.5 million in 2024 (2023: $46.1m), impacted by the launch and NRDL26 inclusion of several competing same-class MET TKIs for 2L METex1427 NSCLC. Results do not reflect full approval in 1L28 setting received in January 2025.
Total Oncology/Immunology consolidated revenue was $363.4 million in 2024 (2023: $528.6m), within guidance of $300 million to $400 million.
- Oncology product consolidated revenue (royalties, manufacturing revenue, promotion and marketing services revenue and commercial milestone) increased 65% (67% at CER) to $271.5 million (2023: $164.2m), driven by FRUZAQLA® and exceeding guidance of 30% to 50% growth.
- Takeda upfront, regulatory milestones and R&D services revenue were $67.0 million (2023: $345.9m), which included recognition of $48.1 million of the $450.0 million upfront and regulatory milestone payments achieved. This compared to recognition of $312.0 million in 2023.
- Other revenue was $24.9 million (2023: $18.5m), including milestone payment of $6.0 million from AstraZeneca following NDA acceptance in China for ORPATHYS® combined with TAGRISSO®.
$630.2 million total consolidated revenue (2023: $838.0m) including Other Ventures of $266.8 million (2023: $309.4m).
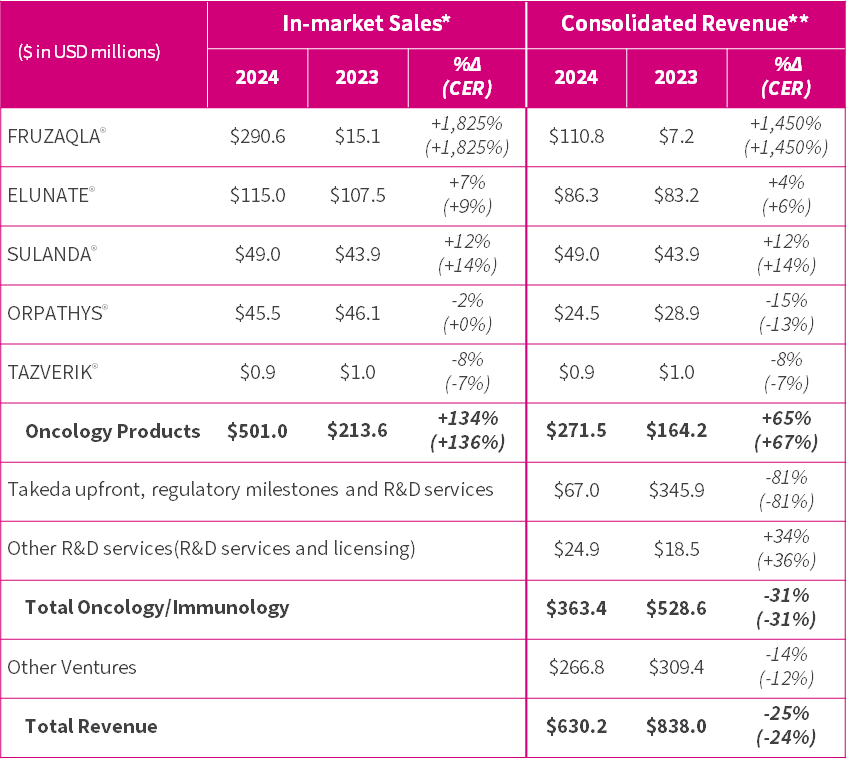
** = FRUZAQLA® represents manufacturing revenue, royalties and commercial milestone paid by Takeda; ELUNATE® represents manufacturing revenue, promotion and marketing services revenue and royalties paid by Lilly to HUTCHMED, and sales to other third parties invoiced by HUTCHMED; ORPATHYS® represents manufacturing revenue and royalties paid by AstraZeneca and sales to other third parties invoiced by HUTCHMED; SULANDA® and TAZVERIK® represent the Company’s sales of the products to third parties.
II. REGULATORY UPDATES
China
- Savolitinib NDA accepted by the NMPA with Priority Review status and Breakthrough Therapy designation for 2L EGFRm NSCLC patients with MET amplification, in combination with TAGRISSO® (osimertinib), in December 2024, triggering a milestone from AstraZeneca.
- Savolitinib sNDA30 approved by the NMPA for 1L and 2L (converted from conditional to full approval) METex14 NSCLC in January 2025.
- Fruquintinib sNDA approved by the NMPA, in combination with TYVYT® (sintilimab), for 2L EMC patients with pMMR status in December 2024.
- Fruquintinib approved in Hong Kong for 3L31 CRC under the new 1+ Mechanism in January 2024, and subsequently the first innovative oncology medicine enlisted with Full Subsidy under the Special Drug category in October 2024.
- Tazemetostat approved in Hong Kong for 3L R/R32 EZH2m33 follicular lymphoma in May 2024.
- Savolitinib approved in Hong Kong for METex14 NSCLC under the 1+ Mechanism in February 2025.
- Tazemetostat NDA accepted by the NMPA with Priority Review status for 3L R/R follicular lymphoma in July 2024.
- Fruquintinib sNDA voluntarily withdrawn for 2L gastric cancer, in combination with paclitaxel, in August 2024, in light of discussions with the NMPA and internal review of current data package.
Ex-China
- Fruquintinib approved in the EU for CRC in June 2024, followed by first European reimbursement in Spain in December 2024, triggering a $10.0 million milestone from Takeda.
- Fruquintinib approved in Japan for CRC in September 2024, followed by pricing approval and launch in November 2024, triggering a milestone from Takeda.
- Fruquintinib approved in Argentina and Switzerland in August 2024, in Canada (also with reimbursement) and the United Kingdom in September 2024, in Australia and Singapore in October 2024, in Israel and the United Arab Emirates in December 2024, and in South Korea in March 2025.
III. LATE-STAGE CLINICAL DEVELOPMENT ACTIVITIES
Savolitinib (ORPATHYS® in China), a highly selective oral inhibitor of MET
- Positive SAVANNAH global pivotal Phase II top-line results for 2L EGFRm NSCLC patients with MET amplification or overexpression, in combination with TAGRISSO® (osimertinib), achieving high, clinically meaningful and durable response rate (NCT03778229).
- Primary endpoint met in SACHI China Phase III interim analysis for 2L EGFRm NSCLC patients with MET amplification (NCT05015608).
- Presented Phase II small randomized controlled study results at AACR34 for 2L EGFRm NSCLC patients with high MET amplification, in combination with TAGRISSO® (osimertinib), showing ORR35 of 63% and median PFS36 of 8.2 months (NCT04606771).
- Continued enrolling SAFFRON global Phase III study for 2L EGFRm NSCLC patients with MET amplification or overexpression (NCT05261399) supporting SAVANNAH; and SANOVO China Phase III study for 1L EGFRm NSCLC patients with MET overexpression (NCT05009836).
Potential upcoming clinical and regulatory milestones for savolitinib:
- Presentation of SAVANNAH and SACHI data at upcoming scientific conferences.
- Complete SACHI NMPA NDA review in late 2025.
- Complete SAFFRON enrollment in the second half of 2025.
- Complete enrollment and potential NDA submission for gastric cancer with MET amplification in the second half of 2025.
Fruquintinib (ELUNATE® in China, FRUZAQLA® outside of China), a highly selective oral inhibitor of VEGFR37
- Presented FRUSICA-1 China pivotal Phase II results at ASCO, in combination with TYVYT®(sintilimab), for previously treated EMC with pMMR status, showing IRC38-assessed confirmed ORR of 35.6%, median PFS of 9.5 months and median OS39 of 21.3 months with a manageable safety profile (NCT03903705). This indication was approved by the NMPA in December 2024.
- Presented FRESCO-2 subgroup analyses for CRC patients at ASCO, biomarker analysis at AACR and quality-of-life analysis at ASCO GI40, showing meaningful quality-adjusted survival benefit, efficacy regardless of prior therapy or sequence as well as CEA41 potentially a predictor of efficacy (NCT04322539).
- Published FRUTIGA China Phase III results in Nature Medicine for 2L gastric cancer, in combination with paclitaxel, and presentations at ASCO, showing statistically significant improvements in ORR and PFS, as well as OS benefits in sub-group without taking subsequent antitumor therapy (NCT03223376).
- Positive result of FRUSICA-2 China Phase III in 2L RCC in March 2025 (NCT05522231).
Sovleplenib (HMPL-523), an investigative and highly selective oral inhibitor of Syk42
- Published ESLIM-01 China Phase III results for adult patients with primary ITP in China in The Lancet Haematology concurrently with presentations at EHA, showing durable response rate of 48.4%, tolerable safety profile and improved quality of life regardless of prior lines of therapies (NCT05029635).
- Presented ESLIM-01 China Phase III long-term results at ASH, showing durable response rate of 51.4% and long-term durable response rate of 59.8% as well as consistent safety profile.
- Published China Phase II results in warm AIHA in China at EHA and in The Lancet Haematology in 2025, demonstrating overall response rate of 66.7% and a favorable safety profile (NCT05535933).
- Initiated ESLIM-02 China Phase III stage in warm AIHA (NCT05535933).
Potential upcoming clinical milestones for sovleplenib:
- Complete ESLIM-01 NMPA NDA review around end 2025 (NCT05029635).
- Complete enrollment of ESLIM-02 Phase III in the second half of 2025 (NCT05535933).
Surufatinib (SULANDA® in China), an oral inhibitor of VEGFR, FGFR43 and CSF-1R44
- Completed enrollment of Phase II part of a China Phase II/III trial for 1L metastatic PDAC45 patients, in combination with AiRuiKa® (camrelizumab), nab-paclitaxel and gemcitabine (NCT06361888). This study was informed in part by an investigator-initiated trial presented at ASCO GI 2024 of a similar combination.
Potential upcoming clinical milestone for surufatinib:
- Data readout of the PDAC Phase II trial in late 2025.
Tazemetostat (TAZVERIK® in Hainan, Macau and Hong Kong), a first-in-class, oral inhibitor of EZH2
- Positive bridging study in 3L follicular lymphoma leading to NDA submission with Priority Review status (NCT05467943).
- Continued enrolling SYMPHONY-1 Phase III China portion of the global study, in combination with lenalidomide and rituximab, in follicular lymphoma patients (NCT04224493).
Potential upcoming clinical milestone for tazemetostat:
- Complete NDA review in China in mid 2025.
Fanregratinib (HMPL-453), a novel, highly selective and potent inhibitor targeting FGFR 1, 2 and 3
- Completed enrollment of registrational China pivotal Phase II for IHCC46 with FGFR2 fusion / rearrangement in March 2025 (NCT04353375).
Ranosidenib (HMPL-306), an investigative and highly selective oral dual-inhibitor of IDH1 and IDH247 enzymes
- Presented and published results from China and US/European Phase I studies at EHA and the journal Med for R/R IDH1/2m48 AML49 patients (NCT04272957, NCT04764474).
- Initiated RAPHAEL China Phase III trial for 2L R/R IDH1/2m AML (NCT06387069).
Other early-stage investigational drug candidates
- Presented pre–clinical and Phase I results at AACR, ASCO and EHA for ERK1/250 inhibitor HMPL-295, third-generation BTK51 inhibitor HMPL-760, Menin inhibitor HMPL-506, and anti-CD38 HMPL-A067.
- Initiated Phase I trial for HMPL-506 in hematological malignancies in China (NCT06387082).
IV. ANTIBODY-TARGETED THERAPY CONJUGATE (ATTC) PLATFORM
New in-house created platform with multiple potential IND52 candidates
Our ATTC next-generation technology platform leverages over 20 years of expertise in targeted therapies with small molecules inhibitors. ATTC drug candidates enrich the next wave of clinical development with potential key advantages over traditional antibody-drug conjugates and/or small molecule medicines:
- Better efficacy through synergistic antibody-small molecule targeted therapy combinations that will target specific mutations; overcome drug resistance and potentially support combinations with other targeted therapies, chemotherapy and immunotherapy, in early-line patient settings.
- Improved safety and prolonged treatment given lower off-tumor or off-target toxicity than small molecules, less myelosuppression and better quality of life than cytotoxin-based conjugates.
- Attractive pharmacokinetics tackles difficult drug targets, enabled by antibody-guided delivery to target sites which will improve bioavailability and reduce drug-drug interactions when compared to oral small molecules inhibitors.
V. COLLABORATION UPDATES
Further progress by Inmagene53 with two candidates discovered by HUTCHMED
- HUTCHMED received 7.5% shareholding interest in Inmagene following the latter’s exercise of an option to exclusively develop, manufacture and commercialize IMG-007, a nondepleting anti-OX40 antibody, and IMG-004, a reversible, non-covalent, highly selective oral BTK inhibitor.
- Inmagene and Ikena Oncology, Inc. (Nasdaq: IKNA) agreed to merge, which is expected to close in mid-2025, subject to closing conditions. HUTCHMED will have an interest in the merged company.
- Inmagene announced positive results of a Phase IIa trial with IMG-007 for atopic dermatitis, showing Week 16 mean change in EASI54 of 77% and EASI-75 response of 54% (NCT05984784). A Phase IIb dose-finding study with a subcutaneous formulation in moderate-to-severe atopic dermatitis is planned.
- Inmagene enrolled a Phase IIa trial with IMG-007 for alopecia areata (NCT06060977), and announced results of a Phase I study with IMG-004, indicating once daily dosing potential (NCT05349097).
VI. OTHER VENTURES
- Other Ventures consolidated revenue is predominantly from the prescription drug distribution business55 in China. It decreased by 14% (12% at CER) to $266.8 million (2023: $309.4m) primarily due to lower COVID-related prescription drug distribution sales in 2024.
- Share of equity in earnings of SHPL, a non-consolidated joint venture, slightly decreased by 2% (increased 1% at CER) to $46.5 million (2023: $47.4m) mainly due to increased clinical trial investment for new products.
- Consolidated net income attributable to HUTCHMED from Other Ventures decreased by 5% (2% at CER) to $47.7 million (2023: $50.3m), due to disposal of consumer products business in December 2023, lower COVID-related prescription drug distribution sales and fluctuation in net income contributed from SHPL.
SHPL Disposal: HUTCHMED entered into share purchase agreements to divest its 45.0% equity interest in SHPL for approximately $608 million in cash, retaining a 5.0% equity interest. It is estimated that HUTCHMED will record a pre-tax gain of approximately $477 million.
VII. SUSTAINABILITY
HUTCHMED is committed to progressively embedding sustainability into all aspects of its operations and creating long-term value for its stakeholders. Continued progress was made in 2024 including:
- Sustainability goals and targets: satisfactory progress made in 11 short- to long-term goals and targets; sustainability performance continued to be incorporated into management’s performance-based remuneration. To prepare for new targets setting, sustainability-related efforts were continually assessed and a target achievement roadmap focused on HUTCHMED’s five sustainability pillars is being developed.
- Enhanced climate actions: based on the 2022 climate risk assessment, HUTCHMED conducted another comprehensive assessment on the potential financial impacts of climate risks and opportunities for HUTCHMED with costs estimated under low-, mid-, and high-emission scenarios. This also prepares it for the latest climate-related disclosure requirements of the HKEX56 and other international disclosure standards.
- Biodiversity assessment: a biodiversity assessment was conducted to understand HUTCHMED’s dependency and impact on nature. Based on the results of the assessment, a Biodiversity Policy was prepared and approved by the Board for public disclosure.
- Supplier ESG57 assessment: this was conducted to understand the sustainability maturity of the supplier base and pave the way for a tailored supplier engagement program in 2025.
- Improvement on ESG ratings: MSCI ESG upgraded the rating of HUTCHMED from BBB to A. ISS ESG upgraded the rating of HUTCHMED from C to C+, which is classified as Prime. Its S&P Global ESG score continued to rise from 48 to 53, placing HUTCHMED in the 90th percentile of the industry. Additionally, HUTCHMED achieved an A- rating and a top quartile score in the Hang Seng Corporate Sustainability Index Series rating, particularly in the areas of environment and governance.
In recognition of its marked improvement in sustainability efforts within the pharmaceutical industry, HUTCHMED was honored with multiple ESG awards in 2024. These efforts will continue to guide HUTCHMED towards a more sustainable future. The 2024 Sustainability Report will be published alongside the 2024 Annual Report in April 2025 and will include further information on sustainability initiatives and performance.
Financial Highlights
Foreign exchange impact: The RMB depreciated against the US dollar by approximately 3% during 2024 on average, which has impacted consolidated financial results as highlighted below.
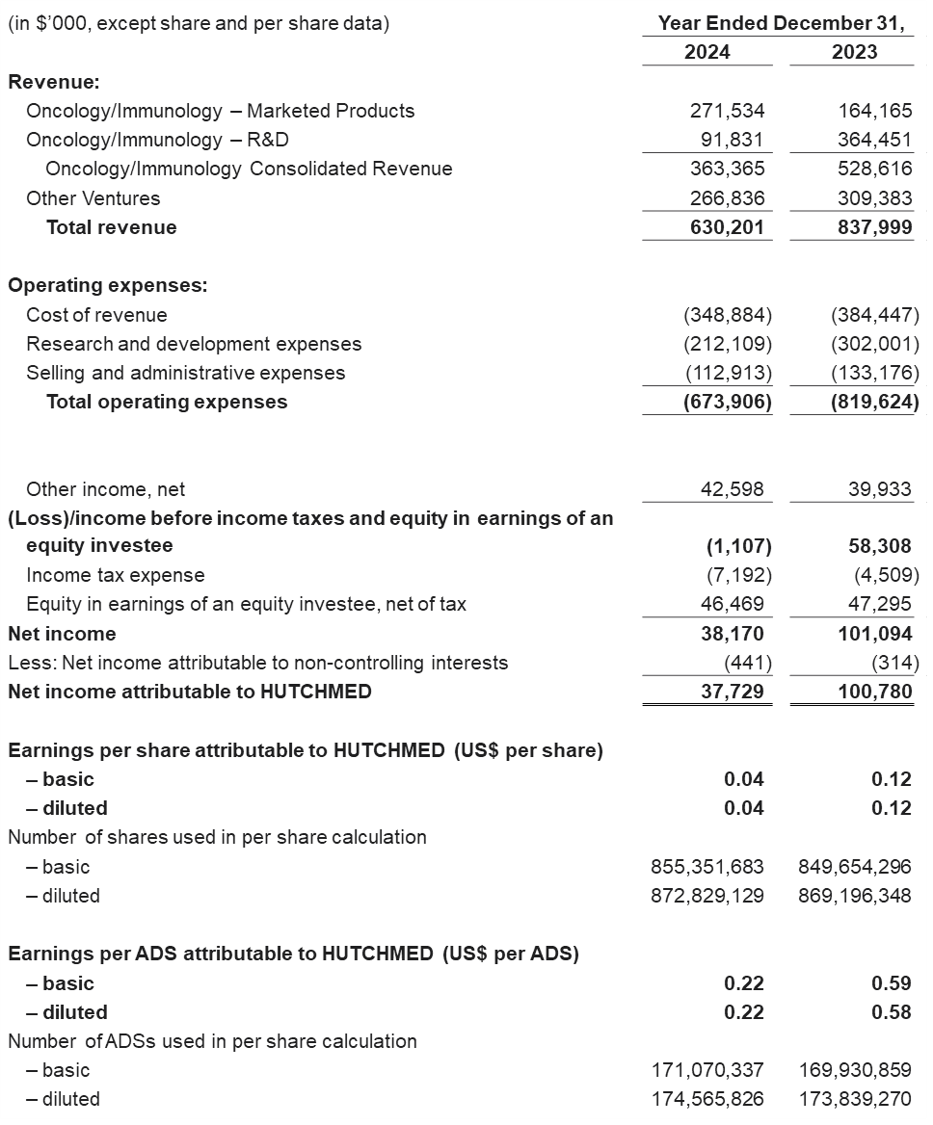
Revenue for the year ended December 31, 2024 was $630.2 million compared to $838.0 million in 2023.
- Oncology/Immunology consolidated revenue amounted to $363.4 million (2023: $528.6m):
- FRUZAQLA® revenue was $110.8 million, reflecting its successful launch since November 2023 comprising royalties, manufacturing revenue and commercial milestone.
- ELUNATE® revenue increased 4% (6% at CER) to $86.3 million (2023: $83.2m) in its sixth year since launch, comprising of manufacturing revenue, promotion and marketing services revenue and royalties, maintaining its leading market share position while weathering greater market competition.
- SULANDA® revenue increased 12% (14% at CER) to $49.0 million (2023: $43.9m) due to continued sales growth after NRDL renewal as brand awareness amongst doctors continues to increase, leading to greater NET patient access and market share.
- ORPATHYS® revenue decreased 15% (13% at CER) to $24.5 million (2023: $28.9m), due to phasing of manufacturing revenue of $10.9 million (2023: $15.1m), and royalties of $13.6 million (2023: $13.8m).
- TAZVERIK® revenue was $0.9 million (2023: $1.0m) mainly from sales in Hainan and Hong Kong.
- Takeda upfront, regulatory milestones and R&D services revenue decreased to $67.0 million (2023: $345.9m, of which $280.0m was the recognized portion of the $400.0 million upfront cash payment received from Takeda in April 2023).
- Other revenue of $24.9 million (2023: $18.5m), primarily related to milestone payment of $6.0 million from AstraZeneca and fees from AstraZeneca and Lilly for development and regulatory activities.
- Other Ventures consolidated revenue decreased 14% (12% at CER) to $266.8 million (2023: $309.4m), primarily as a result of lower COVID-related prescription drug distribution sales in 2024. This excluded non-consolidated revenue at SHPL of $393.5 million (2023: $385.5m).
Net Expenses for 2024 were $592.5 million compared to $737.2 million in 2023, reflecting strong efforts on cost control.
- Cost of Revenue decreased by 9% to $348.9 million (2023: $384.4m), which was mainly due to lower revenue from Other Ventures. Cost of revenue as a percentage of oncology product revenue improved (from 56% in 2023 to 34% in 2024) due to favorable product mix and economies of scale.
- R&D Expenses reduced 30% to $212.1 million (2023: $302.0m), mainly due to restructuring of teams outside of China, with clinical and regulatory expenses in the US and Europe decreasing to $34.5 million (2023: $106.9m). China investment was $177.6 million (2023: $195.1m) which reflects both a decrease in cost for completed studies with NDAs under review and an ongoing commitment to key assets with global potential in our internal pipeline, including the development of the next-generation ATTC platform.
- S&A58 Expenses were $112.9 million (2023: $133.2m), which decreased primarily due to tighter controls over administrative spending $64.3 million (2023: $79.8m) and lower selling expenses $48.6 million (2023: $53.4m) as we realized efficiencies from a salesforce already scaled to support revenue growth.
- Other Items mainly comprised of equity in earnings of SHPL, interest income and expense, FX and taxes, generated net income of $81.4 million (2023: $82.4m).
Net Income attributable to HUTCHMED for 2024 was $37.7 million compared to $100.8 million in 2023.
- The net income attributable to HUTCHMED in 2024 was $0.04 per ordinary share / $0.22 per ADS59, (2023: $0.12 per ordinary share / $0.59 per ADS).
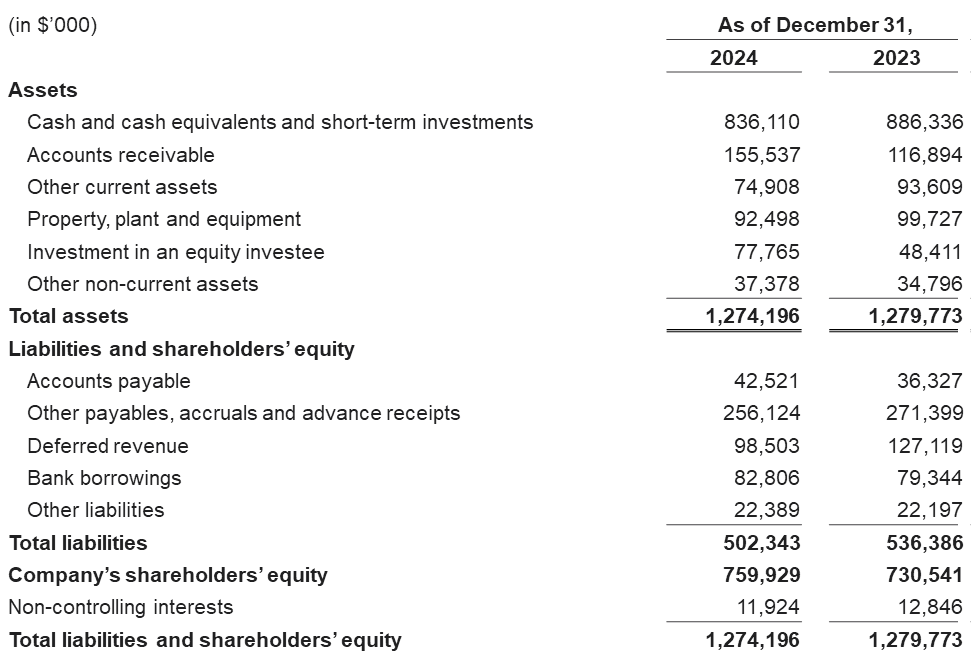
Cash, Cash Equivalents and Short-Term Investments were $836.1 million as of December 31, 2024 compared to $886.3 million as of December 31, 2023.
- Adjusted Group (non-GAAP60) net cash flows excluding financing activities in 2024 were -$19.5 million mainly due to net income attributable to HUTCHMED of $37.7 million offset by changes in working capital of $62.2 million from partner milestones achieved and receivable at the end of 2024 and ongoing recognition of Takeda deferred revenue (2023: $206.7m due to the receipt of $435 million in upfront and milestone payments from Takeda).
- Net cash used in financing activities in 2024 totaled $30.7 million mainly due to purchases for equity awards of $36.1 million (2023: net cash generated from financing activities of $48.7m mainly due to drawdowns of bank borrowings).
FINANCIAL GUIDANCE
HUTCHMED provides full year 2025 guidance for Oncology/Immunology consolidated revenue of $350 million to $450 million. HUTCHMED’s work in 2025 and beyond will be supported by its strong balance sheet. The Company will continue to be financially self-reliant while supporting investments to bring innovative medicines to patients globally.
Shareholders and investors should note that:
- The Company does not provide any guarantee that the statements contained in the financial guidance will materialize or that the financial results contained therein will be achieved or are likely to be achieved; and
- The Company has in the past revised its financial guidance and reference should be made to any announcements published by it regarding any updates to the financial guidance after the date of publication of this announcement.
———
Use of Non-GAAP Financial Measures and Reconciliation – References in this announcement to adjusted Group net cash flows excluding financing activities and financial measures reported at CER are based on non-GAAP financial measures. Please see the “Use of Non-GAAP Financial Measures and Reconciliation” for further information relevant to the interpretation of these financial measures and reconciliations of these financial measures to the most comparable GAAP measures, respectively.
———
FINANCIAL STATEMENTS
HUTCHMED will today file with the US Securities and Exchange Commission its Annual Report on Form 20-F.
FINANCIAL SUMMARY
Condensed Consolidated Balance Sheets Data
Condensed Consolidated Statements of Operations Data
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, and the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Contacts
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
HSBC |
Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
Cavendish |
Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
Unless the context requires otherwise, references in this announcement to the “Group,” the “Company,” “HUTCHMED,” “HUTCHMED Group,” “we,” “us,” and “our,” mean HUTCHMED (China) Limited and its subsidiaries unless otherwise stated or indicated by context.
Past Performance and Forward-Looking Statements
The performance and results of operations of the Group contained within this announcement are historical in nature, and past performance is no guarantee of future results of the Group. This announcement contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements can be identified by words like “will,” “expects,” “anticipates,” “future,” “intends,” “plans,” “believes,” “estimates,” “pipeline,” “could,” “potential,” “first-in-class,” “best-in-class,” “designed to,” “objective,” “guidance,” “pursue,” or similar terms, or by express or implied discussions regarding potential drug candidates, potential indications for drug candidates or by discussions of strategy, plans, expectations or intentions. You should not place undue reliance on these statements. Such forward-looking statements are based on the current beliefs and expectations of management regarding future events, and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. There can be no guarantee that any of our drug candidates will be approved for sale in any market, that any approvals which have been obtained will continue to remain valid and effective in the future, or that the sales of products marketed or otherwise commercialized by HUTCHMED and/or its collaboration partners (collectively, “HUTCHMED’s Products”) will achieve any particular revenue or net income levels. In particular, management’s expectations could be affected by, among other things: unexpected regulatory actions or delays or government regulation generally; the uncertainties inherent in research and development, including the inability to meet our key study assumptions regarding enrollment rates, timing and availability of subjects meeting a study’s inclusion and exclusion criteria and funding requirements, changes to clinical protocols, unexpected adverse events or safety, quality or manufacturing issues; the delay or inability of a drug candidate to meet the primary or secondary endpoint of a study; the delay or inability of a drug candidate to obtain regulatory approval in different jurisdictions or the utilization, market acceptance and commercial success of HUTCHMED’s Products after obtaining regulatory approval; discovery, development and/or commercialization of competing products and drug candidates that may be superior to, or more cost effective than, HUTCHMED’s Products and drug candidates; the impact of studies (whether conducted by HUTCHMED or others and whether mandated or voluntary) or recommendations and guidelines from governmental authorities and other third parties on the commercial success of HUTCHMED’s Products and drug candidates in development; the ability of HUTCHMED to manufacture and manage supply chains, including various third party services, for multiple products and drug candidates; the availability and extent of reimbursement of HUTCHMED’s Products from third-party payers, including private payer healthcare and insurance programs and government insurance programs; the costs of developing, producing and selling HUTCHMED’s Products; the ability to obtain additional funding when needed; the ability to obtain and maintain protection of intellectual property for HUTCHMED’s Products and drug candidates; the ability of HUTCHMED to meet any of its financial projections or guidance and changes to the assumptions underlying those projections or guidance; the successful disposition of its non-core business; global trends toward health care cost containment, including ongoing pricing pressures; uncertainties regarding actual or potential legal proceedings, including, among others, actual or potential product liability litigation, litigation and investigations regarding sales and marketing practices, intellectual property disputes, and government investigations generally; and general economic and industry conditions, including uncertainties regarding the effects of the persistently weak economic and financial environment in many countries, uncertainties regarding future global exchange rates, uncertainties in global interest rates, and geopolitical relations, sanctions and tariffs. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, on AIM and on HKEX. HUTCHMED is providing the information in this announcement as of this date and does not undertake any obligation to update any forward-looking statements as a result of new information, future events or otherwise.
In addition, this announcement contains statistical data and estimates that HUTCHMED obtained from industry publications and reports generated by third-party market research firms. Although HUTCHMED believes that the publications, reports and surveys are reliable, HUTCHMED has not independently verified the data and cannot guarantee the accuracy or completeness of such data. You are cautioned not to give undue weight to this data. Such data involves risks and uncertainties and are subject to change based on various factors, including those discussed above.
Inside Information
This announcement contains inside information for the purposes of Article 7 of Regulation (EU) No 596/2014 (as it forms part of retained EU law as defined in the European Union (Withdrawal) Act 2018).
Medical Information
This announcement contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
Ends
1 In-market sales = total sales to third parties provided by Eli Lilly (ELUNATE®), Takeda (FRUZAQLA®), AstraZeneca (ORPATHYS®) and HUTCHMED (ELUNATE®, SULANDA®, ORPATHYS® and TAZVERIK®).
2 Takeda = Takeda Pharmaceuticals International AG, a subsidiary of Takeda Pharmaceutical Company Limited.
3 SHPL = Shanghai Hutchison Pharmaceuticals Limited.
4 EGFRm = Epidermal growth factor receptor mutated.
5 NSCLC = Non-small cell lung cancer.
6 NDA = New Drug Application.
7 NMPA = China National Medical Products Administration.
8 AstraZeneca = AstraZeneca AB, a subsidiary of AstraZeneca plc.
9 2L = Second-line.
10 RCC = Renal cell carcinoma.
11 ASH = American Society of Hematology.
12 EHA = European Hematology Association.
13 ITP = immune thrombocytopenia purpura.
14 CDE = Centre for Drug Evaluation.
15 ASCO = American Society of Clinical Oncology.
16 EMC = Endometrial cancer.
17 pMMR = Proficient mismatch repair.
18 ATTC = antibody-targeted therapy conjugates.
19 R&D = Research and development.
20 EGFR = Epidermal growth factor receptor.
21 TKI = Tyrosine kinase inhibitor.
22 AIHA = Autoimmune hemolytic anemia.
23 CER = Constant exchange rate. We also report changes in performance at CER which is a non-GAAP measure. Please refer to “Use of Non-GAAP Financial Measures and Reconciliation” for further information relevant to the interpretation of these financial measures and reconciliations of these financial measures to the most comparable GAAP measures.
24 CRC = Colorectal cancer.
25 NET = Neuroendocrine tumor.
26 NRDL = China National Reimbursement Drug List.
27 METex14 = MET exon 14 skipping alteration.
28 1L = First-line.
29 Lilly = Eli Lilly and Company.
30 sNDA = Supplemental New Drug Application.
31 3L = Third-line.
32 R/R = Relapsed and/or refractory.
33 EZH2m = Enhancer of zeste homolog 2 mutated.
34 AACR = American Association for Cancer Research.
35 ORR = Objective response rate.
36 PFS = Progression free survival.
37 VEGFR = Vascular endothelial growth factor receptor.
38 IRC = Independent review committee.
39 OS = Overall survival.
40 ASCO GI = ASCO Gastrointestinal Cancers Symposium.
41 CEA = Carcinoembryonic antigen.
42 Syk = Spleen tyrosine kinase.
43 FGFR = Fibroblast growth factor receptor.
44 CSF-1R = Colony-stimulating factor 1 receptor.
45 PDAC = Pancreatic ductal adenocarcinoma.
46 IHCC = Intrahepatic cholangiocarcinoma.
47 IDH1 and IDH2 = Isocitrate dehydrogenase-1 and isocitrate dehydrogenase-2.
48 IDH1/2m = Isocitrate dehydrogenase-1 OR isocitrate dehydrogenase-2 mutated.
49 AML = Acute myeloid leukemia.
50 ERK = Extracellular signal-regulated kinase.
51 BTK = Bruton’s tyrosine kinase.
52 IND = Investigational new drug application.
53 Inmagene = Inmagene Biopharmaceuticals.
54 EASI = Eczema area and severity index.
55 Distribution business = Shanghai Hutchison Whampoa Pharmaceuticals Sales Limited, formerly Hutchison Whampoa Sinopharm Pharmaceuticals (Shanghai) Company Limited.
56 HKEX = The Main Board of The Stock Exchange of Hong Kong Limited.
57 ESG = Environmental, Social and Governance.
58 S&A = Selling and administrative expenses.
59 ADS = American depositary share.
60 GAAP = Generally Accepted Accounting Principles.
| Announcement release: Mar 19, 2025 (Wed) |
| 7am EDT / 11am GMT / 7pm HKT |
| >> View Announcement<< |
| Presentation webcast & call |
| ⯈ English Session: Mar 19, 2025 (Wed) |
| 8am EDT / 12pm GMT / 8pm HKT |
| Listen to Webcast Replay (English Session) |
| ⯈ Chinese (Putonghua) Session: Mar 20, 2025 (Thu) |
| 12:30am GMT / 8:30am HKT / (8:30pm EDT of Mar 19) |
| Listen to Webcast Replay (Chinese Session) |
Hong Kong, Shanghai & Florham Park, NJ — Wednesday, March 19, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) and Innovent Biologics, Inc. (“Innovent”) (HKEX: 01801), today jointly announce that the FRUSICA-2 Phase II/III clinical trial evaluating fruquintinib in combination with sintilimab as second-line treatment for locally advanced or metastatic renal cell carcinoma (“RCC”) in China has met its primary endpoint of progression free survival (“PFS”) per RECIST 1.1 as assessed by blinded independent central review (BICR).
The combination of fruquintinib and sintilimab received conditional approval from the China National Medical Products Administration (“NMPA”) for the treatment of patients with advanced endometrial cancer with Mismatch Repair proficient (pMMR) tumors that have failed prior systemic therapy and are not candidates for curative surgery or radiation, based on data from the FRUSICA-1 study (NCT03903705).
The FRUSICA-2 study is a randomized, open-label, active-controlled study to evaluate the efficacy and safety of fruquintinib in combination with sintilimab versus axitinib or everolimus monotherapy for the second-line treatment of advanced RCC (NCT05522231). In addition to the primary endpoint PFS, the combination also demonstrated improvements in secondary endpoints including objective response rate (“ORR”) and duration of response (“DoR”). Full results will be submitted for presentation at an upcoming scientific conference.
Prof Dingwei Ye of Fudan University Shanghai Cancer Center and the co-leading Principal Investigator of the FRUSICA-2 study, said, “The rapid advancements in targeted therapies, immunotherapies, and their combination regimens have led to a significant evolution in the treatment landscape for advanced renal cell carcinoma. Targeted therapy remains an indispensable and crucial component in systemic treatment of advanced RCC in China. Optimizing the selection of targeted therapy, either as monotherapy or in combination with immunotherapy, for individual patients is a key focus of clinical interest. The results from the FRUSICA‑2 study underscore the potential of the fruquintinib and sintilimab combination to address the pressing medical needs of patients with this challenging disease.”
Prof Zhisong He of Peking University First Hospital and the co-leading Principal Investigator of the FRUSICA-2 study, said, “The positive results from this Phase III study of the fruquintinib and sintilimab combination represent a significant advancement in the treatment of advanced renal cell carcinoma. We are optimistic about the clinical implications of the findings as we strive to provide more effective treatment options for patients who may not have had adequate responses to previous therapies.”
“The encouraging results from our study provide clear evidence for the combination of fruquintinib and sintilimab as a viable new treatment option for advanced renal cell carcinoma patients who have progressed on previous therapy. This not only reaffirms our commitment to advancing cancer therapies but also represents an important step forward in addressing unmet medical needs within this patient population,” said Dr Michael Shi, Head of R&D and Chief Medical Officer of HUTCHMED. “I extend my heartfelt gratitude to the patients and investigators who participated in this research; their contributions have been vital to our success. We look forward to sharing detailed findings with regulatory authorities and progressing toward NDA filings in the coming months.”
“We are encouraged by the positive results in the FRUSICA-2 clinical trial. These outcomes not only underscore the great potential of the combination therapy of sintilimab and fruquintinib but also bring new hope to previously-treated patients with advanced renal cell carcinoma. We look forward to working closely with HUTCHMED to jointly advance the registrational communication of this innovative combo therapy and make it available to patients as soon as possible,” said Dr Hui Zhou, Senior Vice President of Innovent.
About Kidney Cancer and RCC
It is estimated that approximately 435,000 new patients were diagnosed with kidney cancer worldwide in 2022.[1] In China, an estimated 74,000 new patients were diagnosed with kidney cancer in 2022.[2] Approximately 90% of kidney tumors are RCC.
About Fruquintinib
Fruquintinib is a selective oral inhibitor of all three vascular endothelial growth factor (“VEGF”) receptors (VEGFR-1, -2 and -3). VEGFR inhibitors play a pivotal role in inhibiting tumor angiogenesis. Fruquintinib was designed to have enhanced selectivity that limits off-target kinase activity, allowing for drug exposure that achieves sustained target inhibition and flexibility for potential use as part of a combination therapy.[3]
About Fruquintinib Approvals
Fruquintinib is co-developed and co-marketed in China by HUTCHMED and Eli Lilly and Company under the brand name ELUNATE®. It is approved for the treatment of patients with metastatic colorectal cancer who have previously received fluoropyrimidine, oxaliplatin and irinotecan-based chemotherapy, and those who have previously received or are not suitable for receiving anti-VEGF therapy or anti-epidermal growth factor receptor (EGFR) therapy (RAS wild-type) in China. It was included in the China National Reimbursement Drug List (NRDL) in January 2020. Since its launch in China, over 100,000 patients with colorectal cancer have been treated with fruquintinib.
The combination of ELUNATE® (fruquintinib) and TYVYT® (sintilimab injection) has conditional approval in China for the treatment of patients with advanced endometrial cancer with Mismatch Repair proficient (pMMR) tumors that have failed prior systemic therapy and are not candidates for curative surgery or radiation.
Takeda holds the exclusive worldwide license to further develop, commercialize, and manufacture fruquintinib outside mainland China, Hong Kong and Macau, marketing it under the brand name FRUZAQLA®. Fruquintinib received approval for the treatment of previously treated metastatic colorectal cancer in the US in November 2023, in the EU in June 2024, in Switzerland and Argentina in August 2024, in Canada, Japan and the United Kingdom in September 2024, in Australia and Singapore in October 2024 and in Israel and the United Arab Emirates in December 2024. Regulatory applications are progressing in many other jurisdictions.
The global regulatory submissions are based on data from two large, randomized, controlled Phase III trials in colorectal cancer, the global, multi-regional FRESCO-2 trial and the FRESCO trial conducted in China, showing consistent benefit among a total of 734 metastatic colorectal cancer patients treated with fruquintinib. Safety profiles were consistent across trials. Results from the FRESCO-2 trial were published in The Lancet in June 2023,[4] while results from the FRESCO trial were published in The Journal of the American Medical Association, JAMA.[5]
The safety and efficacy of fruquintinib for the following investigational uses have not been established and there is no guarantee that it will receive health authority approval or become commercially available in any country for the uses being investigated:
About Fruquintinib for Second-line Treatment of RCC
The U.S. Food and Drug Administration (FDA) has approved five immune-oncology combination therapies for the first-line treatment of advanced RCC. However, only one immune-oncology combination therapy has been approved in China for advanced RCC patients classified as having intermediate or poor risk by the International mRCC Database Consortium (IMDC). Single-agent targeted therapy continues to be one of the primary choices for first-line treatment of advanced RCC in China. Notably, advanced RCC patients who have experienced failure with single-agent targeted therapy previously still indicate an unmet medical need.
Results from a proof-of-concept Phase Ib/II study of fruquintinib plus sintilimab were published in Targeted Oncology in January 2025. The combination demonstrated promising efficacy and a tolerable safety profile in this setting. At the data cutoff of October 9, 2024, all 20 enrolled previously treated patients were evaluable for efficacy, with a median follow-up duration of 45.7 months. The confirmed ORR was 60.0% and DCR was 85.0%. Median DoR was 13.9 months and median PFS was 15.9 months. Overall survival (“OS”) was not reached, and the 36-month OS rate was 58.3%.[6]
About Sintilimab
Sintilimab, marketed as TYVYT® (sintilimab injection) in China, is a PD-1 immunoglobulin G4 monoclonal antibody co-developed by Innovent and Eli Lilly and Company. Sintilimab is a type of immunoglobulin G4 monoclonal antibody, which binds to PD-1 molecules on the surface of T-cells, blocks the PD-1 / PD-Ligand 1 (PD-L1) pathway, and reactivates T-cells to kill cancer cells. [7]
In China, sintilimab has been approved and included in the updated NRDL for seven indications. The updated NRDL reimbursement scope for TYVYT® (sintilimab injection) includes:
- For the treatment of relapsed or refractory classic Hodgkin’s lymphoma after two lines or later of systemic chemotherapy;
- For the first-line treatment of unresectable locally advanced or metastatic non-squamous non-small cell lung cancer lacking EGFR or ALK driver gene mutations;
- For the treatment of patients with EGFR-mutated locally advanced or metastatic non-squamous non-small cell lung cancer who progressed after EGFR-TKI therapy;
- For the first-line treatment of unresectable locally advanced or metastatic squamous non-small cell lung cancer;
- For the first-line treatment of unresectable or metastatic hepatocellular carcinoma with no prior systematic treatment;
- For the first-line treatment of unresectable locally advanced, recurrent or metastatic esophageal squamous cell carcinoma;
- For the first-line treatment of unresectable locally advanced, recurrent or metastatic gastric or gastroesophageal junction adenocarcinoma.
Furthermore, sintilimab’s eighth indication, in combination with fruquintinib for the treatment of patients with advanced endometrial cancer with pMMR tumors that have failed prior systemic therapy and are not candidates for curative surgery or radiation, was conditional approved by the NMPA in December 2024. And the NDA for sintilimab in combination with ipilimumab as neoadjuvant treatment for resectable MSI-H/dMMR colon cancer is under the NMPA review and has been granted Priority Review designation.
In addition, three clinical studies of sintilimab have met their primary endpoints:
- Phase 2 study of sintilimab monotherapy as second-line treatment of esophageal squamous cell carcinoma;
- Phase 3 study of sintilimab monotherapy as second-line treatment for squamous non-small cell lung cancer with disease progression following platinum-based chemotherapy;
- Phase 2/3 study of sintilimab in combination with fruquintinib versus axitinib or everolimus monotherapy for the second-line treatment of advanced RCC.
Statement: Innovent does not recommend the use of any unapproved drug(s)/indication(s).
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery, global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception, HUTCHMED has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved around the world including in the US, Europe and Japan. For more information, please visit www.hutch-med.com or follow us on LinkedIn.
About Innovent
Innovent is a leading biopharmaceutical company founded in 2011 with the mission to empower patients worldwide with affordable, high-quality biopharmaceuticals. The company discovers, develops, manufactures and commercializes innovative medicines that target some of the most intractable diseases. Its pioneering therapies treat cancer, cardiovascular and metabolic, autoimmune and eye diseases. Innovent has launched 15 products in the market. It has 3 new drug applications under regulatory review, 3 assets in Phase III or pivotal clinical trials and 16 more molecules in early clinical stage. Innovent partners with over 30 global healthcare companies, including Lilly, Sanofi, Incyte, Adimab, LG Chem and MD Anderson Cancer Center.
Guided by the motto, “Start with Integrity, Succeed through Action,” Innovent maintains the highest standard of industry practices and works collaboratively to advance the biopharmaceutical industry so that first-rate pharmaceutical drugs can become widely accessible. For more information, visit www.innoventbio.com, or follow Innovent on Facebook and LinkedIn.
Statement:
(1)Innovent does not recommend the use of any unapproved drug (s)/indication(s).
(2)Ramucirumab (Cyramza®) and Selpercatinib (Retsevmo®) and Pirtobrutinib (Jaypirca®) were developed by Eli Lilly and Company.
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the therapeutic potential of the fruquintinib and sintilimab combination for the treatment of patients with RCC and the further clinical development of the fruquintinib and sintilimab combination in this and other indications. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding the timing and outcome of clinical studies and the sufficiency of clinical data to support NDA approval of the fruquintinib and sintilimab combination for the treatment of patients with RCC or other indications in China or other jurisdictions, its potential to gain approvals from regulatory authorities on an expedited basis or at all, the safety profile of the fruquintinib and sintilimab combination, HUTCHMED’s ability to fund, implement and complete its further clinical development and commercialization plans for fruquintinib and the timing of these events. In addition, as certain studies rely on the use of other drug products such as sintilimab as combination therapeutics with fruquintinib, such risks and uncertainties include assumptions regarding the safety, efficacy, supply and continued regulatory approval of these therapeutics. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the U.S. Securities and Exchange Commission, on The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
CONTACTS
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
HSBC |
Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
Cavendish |
Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
[1] The Global Cancer Observatory, kidney cancer fact sheet. https://gco.iarc.who.int/media/globocan/factsheets/cancers/29-kidney-fact-sheet.pdf. Accessed February 19, 2025.
[2] The Global Cancer Observatory, China fact sheet. https://gco.iarc.who.int/media/globocan/factsheets/populations/160-china-fact-sheet.pdf. Accessed February 19, 2025.
[3] Sun Q, et al. Discovery of fruquintinib, a potent and highly selective small molecule inhibitor of VEGFR 1, 2, 3 tyrosine kinases for cancer therapy. Cancer Biol Ther. 2014;15(12):1635-45. doi: 10.4161/15384047.2014.964087.
[4] Dasari NA, et al. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO‑2): an international, multicentre, randomised, double‑blind, Phase III study. Lancet. 2023;402(10395):41‑53. doi:10.1016/S0140‑6736(23)00772‑9.
[5] Li J, et al. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA. 2018;319(24):2486-2496. doi:10.1001/jama.2018.7855.
[6] Xu H, et al. Fruquintinib Plus Sintilimab in Patients with Treatment‑Naïve and Previously Treated Advanced Renal Cell Carcinoma: Results from a Phase Ib/II Clinical Trial. Targeted Oncology. 2025; 20:113–125. doi.org/10.1007/s11523-024-01120-6.
[7] Wang J, et al. Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. mAbs 2019;11(8): 1443-1451. doi: 10.1080/19420862.2019.1654303.
HUTCHMED (China) Limited (“HUTCHMED”) notes the below text, which is from a voluntary announcement released to the Stock Exchange of Hong Kong Limited on March 14, 2025. The text relates to the adoption of the 2025 Long Term Incentive Plan with effect from April 24, 2025.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
CONTACTS
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
HSBC |
Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
Cavendish |
Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
Hong Kong, Shanghai & Florham Park, NJ — Friday, March 14, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM: HCM; SEHK:13) announces that the non-performance based awards granted under the Long Term Incentive Plan (“LTIP”) on March 13, 2024 to the following person discharging managerial responsibilities were vested on March 13, 2025:-
| Award Holder | Number of ordinary shares | |
| Dr Weiguo Su (Executive Director, Chief Executive Officer and Chief Scientific Officer) | 19,913 |
The notification set out below is provided in accordance with the requirements of the UK Market Abuse Regulation.
Dr Weiguo Su
| 1 | Details of the person discharging managerial responsibilities/person closely associated | |||||
| a) | Name | Dr Weiguo Su | ||||
| 2 | Reason for the notification | |||||
| a) | Position/status | Executive Director, Chief Executive Officer and Chief Scientific Officer | ||||
| b) | Initial notification/Amendment | Initial notification | ||||
| 3 | Details of the issuer, emission allowance market participant, auction platform, auctioneer or auction monitor | |||||
| a) | Name | HUTCHMED (China) Limited | ||||
| b) | LEI | 2138006X34YDQ6OBYE79 | ||||
| 4 | Details of the transaction(s): section to be repeated for (i) each type of instrument; (ii) each type of transaction; (iii) each date; and (iv) each place where transactions have been conducted | |||||
| a) | Description of the financial instrument, type of instrument Identification code |
Ordinary Shares of US$0.10 Ordinary Share with DI ISIN: KYG4672N1016 |
||||
| b) | Nature of the transaction | Vesting of awards granted on March 13, 2024 under HUTCHMED’s LTIP | ||||
| c) | Price(s) and volume(s) |
|
||||
| d) | Aggregated information
|
N/A | ||||
| e) | Date of the transaction | 2025-03-13 | ||||
| f) | Place of the transaction | Outside a trading venue | ||||
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery, global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception, HUTCHMED has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
CONTACTS
| Investor Enquiries | +852 2121 8200 / ir@hutch-med.com |
| Media Enquiries | |
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
| Panmure Liberum | Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
| HSBC | Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
| Cavendish | Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
| Date: | Monday, March 31, 2025 |
| Time: | 3:00 pm Hong Kong Time (8:00 am London Time) |
| Location: | 47th Floor, Cheung Kong Center, 2 Queen’s Road Central, Hong Kong |
| Online: | https://meetings.computershare.com/Hutchmed2025EGM |
Hong Kong, Shanghai & Florham Park, NJ — Thursday, March 6, 2025: HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today announces that it has completed enrollment of its a Phase II trial of fanregratinib (HMPL-453) for intrahepatic cholangiocarcinoma (“IHCC”) patients with fibroblast growth factor receptor (“FGFR”)2 fusion/rearrangement.
The study is a single-arm, multi-center, open-label, Phase II registration study to evaluate the efficacy, safety and pharmacokinetic of fanregratinib in treating advanced IHCC patients with FGFR2 fusion/rearrangement. Primary endpoint is objective response rate (ORR). Secondary endpoints include progression-free survival (PFS), disease control rate (DCR), duration of response (DoR) and overall survival (OS). A total of 87 patients were enrolled into the registration phase of the study. Additional details may be found at clinicaltrials.gov using identifier NCT04353375.
The first patient received the first dose in March 2023 and HUTCHMED expects to announce topline results from the study around the end of 2025. If favorable, the results could enable a New Drug Application submission to China’s National Medical Products Administration (NMPA).
About Fanregratinib
Fanregratinib (HMPL‑453) is a novel, highly selective and potent inhibitor targeting FGFR 1, 2 and 3. Aberrant FGFR signaling has been found to be a driving force in tumor growth, promotion of angiogenesis and resistance to anti-tumor therapies. Abnormal FGFR gene alterations are believed to be the drivers of tumor cell proliferation in several solid tumor settings.
HUTCHMED currently retain all rights to fanregratinib worldwide.
About IHCC with FGFR2 Fusion/Rearrangement
IHCC is one of the subtypes of primary bile duct cancer. In China, an estimated 61,900 newly diagnosed IHCC occurred in 2015 and the overall IHCC incidence increased by 9.2% per year between 2006 and 2015.[1] FGFR2 fusion has been reported to have a prevalence of 10-15% in IHCC patients.[2],[3]
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events, including its expectations regarding the therapeutic potential of fanregratinib, the further clinical development for fanregratinib, its expectations as to whether any studies on fanregratinib would meet their primary or secondary endpoints, and its expectations as to the timing of the completion and the release of results from such studies. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, assumptions regarding enrollment rates and the timing and availability of subjects meeting a study’s inclusion and exclusion criteria; changes to clinical protocols or regulatory requirements; unexpected adverse events or safety issues; the ability of fanregratinib, including as a combination therapy, to meet the primary or secondary endpoint of a study, to obtain regulatory approval in different jurisdictions and to gain commercial acceptance after obtaining regulatory approval; the potential market of fanregratinib for a targeted indication and the sufficiency of funding. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, The Stock Exchange of Hong Kong Limited and on AIM. HUTCHMED undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
CONTACTS
| Investor Enquiries | +852 2121 8200 / ir@hutch-med.com |
| Media Enquiries | |
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
| Panmure Liberum | Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
| HSBC | Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
| Cavendish | Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
[1] An L, Zheng R, Zhang S, et al. Hepatocellular carcinoma and intrahepatic cholangiocarcinoma incidence between 2006 and 2015 in China: estimates based on data from 188 population-based cancer registries. Hepatobiliary Surg Nutr. 2023 Feb 28;12(1):45-55.
[2] Arai Y, Totoki Y, Hosoda F, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology. 2014;59:1427–34.
[3] Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003–10.
Hong Kong, Shanghai & Florham Park, NJ — Wednesday, March 5, 2025: HUTCHMED (China) Limited (“HUTCHMED” or the “Company”) (Nasdaq/AIM:HCM, HKEX:13) today announces that Mr Wong Tak Wai (Mr Alvin Wong) is appointed as an Independent Non-executive Director and a member of the Audit Committee of the Company with effect from March 6, 2025.
Mr Wong has over 35 years of extensive experience in accounting, auditing and corporate finance. He has acted in a pivotal role in assisting companies with their stock exchange listings and has been instrumental in completing numerous mergers and acquisitions. After a distinguished career spanning more than three decades, Mr Wong retired as a partner of PricewaterhouseCoopers in 2017.
Dr Dan Eldar, Chairman of HUTCHMED, said “On behalf of the Board, I would like to extend a warm welcome to Mr Wong to the Company. We believe that his extensive experience in finance and accounting will greatly contribute to the strategic growth and financial stewardship of the Company.”
Mr Wong, aged 68, is currently a non-executive director of Melbourne Enterprises Limited (HKEX: 158). He was the president and a council member of the Hong Kong Institute of Certified Public Accountants (“HKICPA”), chairman of the HKICPA auditing standards committee, and a member of various committees of the International Federation of Accountants. He was also a member of the Sustainable Agricultural Development Fund Advisory Committee.
Mr Wong holds a Bachelor of Commerce degree from University of Otago, New Zealand and is a Fellow of the HKICPA and an Associate of the Institute of Chartered Accountants of Australia and New Zealand.
Mr Wong currently holds or has held in the past five years the following directorships / partnerships:
| Current Directorships / Partnerships: | Previous Directorships / Partnerships in the last five years: |
|
Chinachem Group Holdings Limited Melbourne Enterprises Limited Pondbury Investments Limited |
Estate Of Wang Teh Huei Ltd Estate Of Wang Teh Huei (No. 2) Ltd Vipin Limited Wyberton Limited |
Save for the appointments listed above, Mr Wong has held no other directorships or partnerships during the period of five years prior to his appointment as a director of HUTCHMED. He does not have any relationship with any Directors, senior management or substantial or controlling shareholders of HUTCHMED. Mr Wong has confirmed (a) his independence as regards each of the factors for independence referred to in Rule 3.13(1) to (8) of the Rules Governing the Listing of Securities on The Stock Exchange of Hong Kong Limited (“HK Listing Rules”); (b) that he had no past or present financial or other interest in the business of the Company or its subsidiaries or any connection with any core connected person (as defined in the HK Listing Rules) of the Company; and (c) that there are no other factors that may affect his independence at the time of his appointment.
The initial term of the appointment of Mr Wong as an Independent Non-executive Director of the Company shall end at the next following annual general meeting of the Company, subject to retirement in accordance with the Articles of Association of the Company and applicable legal and regulatory requirements, and thereafter for successive periods of 12 months, unless he is not re-elected at the next following annual general meeting or his appointment is otherwise terminated earlier by either party in writing. The director’s fees of Mr Wong as an Independent Non-executive Director and member of the Audit Committee of the Company under his appointment letter are US$76,000 and US$13,500 per annum respectively, which were determined by the Board with reference to the director’s duties and responsibilities and prevailing market conditions. Such fees are subject to review from time to time and proration for any incomplete year of service.
Mr Wong does not have any interest in any shares of the Company within the meaning of Part XV of the Securities and Futures Ordinance (Cap. 571 of the Laws of Hong Kong).
Save for the information disclosed above, there is no other information in relation to Mr Wong that is required to be disclosed pursuant to Rule 17 and Schedule 2(g) of the AІM Rules for Companies or Rule 13.51(2) of the HK Listing Rules, and there are no other matters concerning the appointment of Mr Wong that are required to be brought to the attention of the shareholders of HUTCHMED.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery, global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception, HUTCHMED has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
Forward-Looking Statements
This announcement contains forward-looking statements within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect HUTCHMED’s current expectations regarding future events. Forward-looking statements involve risks and uncertainties. Such risks and uncertainties include, among other things, the risk that current or future appointees to HUTCHMED’s board of directors are not effective in their respective positions, the difficulty in locating and recruiting suitable candidates for its board of directors and the management difficulties which may arise from changes in HUTCHMED’s board of directors. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. For further discussion of these and other risks, see HUTCHMED’s filings with the US Securities and Exchange Commission, on AІM and with The Stock Exchange of Hong Kong Limited. HUTCHMED undertakes no obligation to update or revise the information contained in this announcement, whether as a result of new information, future events or circumstances or otherwise.
CONTACTS
| Investor Enquiries | +852 2121 8200 / ir@hutch-med.com |
| Media Enquiries | |
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
| Panmure Liberum | Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
| HSBC | Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
| Cavendish | Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |
HUTCHMED (China) Limited (“HUTCHMED”) notes the below text, which is from an announcement released to the Stock Exchange of Hong Kong Limited on March 2, 2025 pursuant to Chapter 14 of the Rules Governing the Listing of Securities on The Stock Exchange of Hong Kong Limited.
The announcement provides an update on the proposed divestment of HUTCHMED’s 45% equity interest in Shanghai Hutchison Pharmaceuticals Limited (“SHPL”) as described in the RNS announcements dated January 2, 2025. As previously announced, the transaction includes the proposed disposal of shares representing a 35% equity interest in SHPL to GP Health Service Capital Co., Ltd (“GP Health Service Capital”), which in turn has the right to designate (i) a party to purchase no more than 10% equity interest in SHPL, and (ii) a fund established by GP Health Service Capital as manager and one of the general partners to purchase the remaining shares.
The below text describes the investment funds that have been designated by GP Health Service Capital, and provides an update on the date when the Extraordinary General Meeting Circular is expected to be dispatched to shareholders.
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. Since inception it has focused on bringing drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also approved in the US, Europe and Japan. For more information, please visit: www.hutch‑med.com or follow us on LinkedIn.
CONTACTS
Investor Enquiries |
+852 2121 8200 / ir@hutch-med.com |
Media Enquiries |
|
| FTI Consulting – | +44 20 3727 1030 / HUTCHMED@fticonsulting.com |
| Ben Atwell / Alex Shaw | +44 7771 913 902 (Mobile) / +44 7779 545 055 (Mobile) |
| Brunswick – Zhou Yi | +852 9783 6894 (Mobile) / HUTCHMED@brunswickgroup.com |
Panmure Liberum |
Nominated Advisor and Joint Broker |
| Atholl Tweedie / Freddy Crossley / Rupert Dearden | +44 20 7886 2500 |
HSBC |
Joint Broker |
| Simon Alexander / Alina Vaskina / Arnav Kapoor | +44 20 7991 8888 |
Cavendish |
Joint Broker |
| Geoff Nash / Nigel Birks | +44 20 7220 0500 |